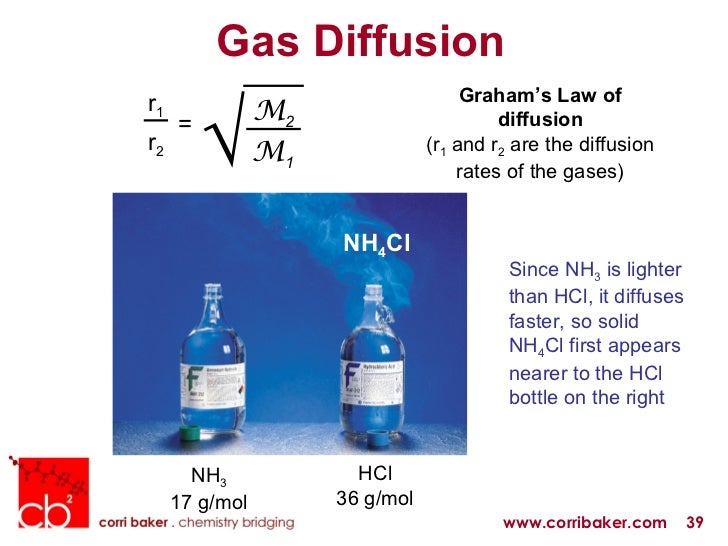
Lighter Gas Particles Diffuse Less Rapidly . the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. The concentration gradient (the increase or decrease in concentration from one point to another); the lightest gases have a wider distribution of speeds and the highest average speeds. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the diffusion rate depends on several factors: effectively, this means that only one particle passes through at a time.
from www.slideshare.net
the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the lightest gases have a wider distribution of speeds and the highest average speeds. the diffusion rate depends on several factors: The concentration gradient (the increase or decrease in concentration from one point to another); if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. effectively, this means that only one particle passes through at a time. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse.
5. Gases
Lighter Gas Particles Diffuse Less Rapidly the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. the lightest gases have a wider distribution of speeds and the highest average speeds. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. The concentration gradient (the increase or decrease in concentration from one point to another); If a mixture of gases is placed in a container with porous walls, the gases effuse. the diffusion rate depends on several factors: if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. effectively, this means that only one particle passes through at a time. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls.
From www.slideserve.com
PPT Unit 9 The Gas Laws PowerPoint Presentation, free download ID1901158 Lighter Gas Particles Diffuse Less Rapidly effectively, this means that only one particle passes through at a time. the lightest gases have a wider distribution of speeds and the highest average speeds. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the lighter gases pass through the small openings. Lighter Gas Particles Diffuse Less Rapidly.
From www.youtube.com
gas diffusion animation by Russell Kightley YouTube Lighter Gas Particles Diffuse Less Rapidly If a mixture of gases is placed in a container with porous walls, the gases effuse. The concentration gradient (the increase or decrease in concentration from one point to another); if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. if a mixture of gases. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideshare.net
5. Gases Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. The concentration gradient (the increase or decrease in concentration from one point. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
Unit 7 Chapter 2 & 3 Matter cont. ppt download Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. effectively, this means that only one particle passes through at a time. the diffusion rate depends on several factors: If a mixture of gases is placed in a container with porous walls, the gases. Lighter Gas Particles Diffuse Less Rapidly.
From saylordotorg.github.io
Gases Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); effectively, this means that only one particle passes through at a time. the diffusion rate depends on several factors: if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. . Lighter Gas Particles Diffuse Less Rapidly.
From www.slideserve.com
PPT Section 10.5 The Molecular Theory PowerPoint Presentation ID2710837 Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the lighter gases pass through the small openings more rapidly (at a. Lighter Gas Particles Diffuse Less Rapidly.
From www.youtube.com
Motion of particles in gases // animated video MotionInGases MotionOfParticlesInGases YouTube Lighter Gas Particles Diffuse Less Rapidly the diffusion rate depends on several factors: the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. The concentration gradient (the increase or decrease in. Lighter Gas Particles Diffuse Less Rapidly.
From www.sciencefacts.net
Diffusion Definition and How Does it Occur (with Diagram) Lighter Gas Particles Diffuse Less Rapidly the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the lightest gases have a wider distribution of speeds and the highest average speeds. the diffusion rate depends on several factors: If a mixture of gases is placed in a container with porous walls, the. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
STATES OF MATTER. ppt download Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the diffusion rate depends on several factors: the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. if a mixture of. Lighter Gas Particles Diffuse Less Rapidly.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases Owlcation Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse. the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure.. Lighter Gas Particles Diffuse Less Rapidly.
From circuitlibsmoring.z21.web.core.windows.net
Particle Model Motion Diagram Lighter Gas Particles Diffuse Less Rapidly the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls,. Lighter Gas Particles Diffuse Less Rapidly.
From www.researchgate.net
Schematic of the movement of gas molecules a normal gas diffusion and... Download Scientific Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous. Lighter Gas Particles Diffuse Less Rapidly.
From eduinput.com
Diffusion Explained Types, Examples and Factors Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse. the diffusion rate depends on several factors: the lighter gases pass through the small openings more rapidly (at. Lighter Gas Particles Diffuse Less Rapidly.
From diagramlibkutshase6.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. effectively, this means that only one particle passes through at a time. the diffusion rate depends on several factors: the law states that the rate of diffusion of a gas is inversely proportional. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
Chapter 12 States of Matter. ppt download Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the lightest gases have a wider distribution of speeds and the highest average speeds. if a mixture of gases is. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideserve.com
PPT Gases and the Atmosphere PowerPoint Presentation, free download ID4200560 Lighter Gas Particles Diffuse Less Rapidly if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse. the law states that the rate of diffusion of a gas is inversely proportional to the square root of. Lighter Gas Particles Diffuse Less Rapidly.
From mungfali.com
Solids Liquids Gases Chart Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); the diffusion rate depends on several factors: effectively, this means that only one particle passes through at a time. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideshare.net
5. Gases Lighter Gas Particles Diffuse Less Rapidly If a mixture of gases is placed in a container with porous walls, the gases effuse. the diffusion rate depends on several factors: The concentration gradient (the increase or decrease in concentration from one point to another); the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
Equipment Gas pressure and diffusion reading handout ppt download Lighter Gas Particles Diffuse Less Rapidly If a mixture of gases is placed in a container with porous walls, the gases effuse. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
Mean Free Path, Diffusion and Effusion of Gases, and Real Gases ppt download Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. effectively, this means that only one particle passes through at a time. if a mixture of gases is placed in. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
Molecular Theory ppt download Lighter Gas Particles Diffuse Less Rapidly the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. The concentration gradient (the increase or decrease in concentration from one point to another); the lightest gases have a wider distribution of speeds and the highest average speeds. If a mixture of gases is placed in. Lighter Gas Particles Diffuse Less Rapidly.
From courses.lumenlearning.com
8.3 Gases and Pressure The Basics of General, Organic, and Biological Chemistry Lighter Gas Particles Diffuse Less Rapidly the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the lightest gases have a wider distribution of speeds and the highest. Lighter Gas Particles Diffuse Less Rapidly.
From slideplayer.com
P2 Lesson 1 Solids liquids and gases Todays lesson State the distinguishing properties of Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse. effectively, this means that only. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Liquids and Solids PowerPoint Lighter Gas Particles Diffuse Less Rapidly the diffusion rate depends on several factors: if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. if a mixture of gases is placed. Lighter Gas Particles Diffuse Less Rapidly.
From schoolbag.info
Figure 8.8. Diffusion of Solutes in a Solvent Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. effectively, this means that only one particle passes through at a time. the diffusion rate depends on several factors: the. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideshare.net
Behaviour of liquids and gases Lighter Gas Particles Diffuse Less Rapidly effectively, this means that only one particle passes through at a time. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the. Lighter Gas Particles Diffuse Less Rapidly.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Lighter Gas Particles Diffuse Less Rapidly the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the diffusion rate depends on several factors: if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases. Lighter Gas Particles Diffuse Less Rapidly.
From spmchemistry.blog.onlinetuition.com.my
Diffusion in Gas SPM Chemistry Lighter Gas Particles Diffuse Less Rapidly If a mixture of gases is placed in a container with porous walls, the gases effuse. effectively, this means that only one particle passes through at a time. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the lighter gases pass through the. Lighter Gas Particles Diffuse Less Rapidly.
From chem.libretexts.org
7.2 The Gas Laws Chemistry LibreTexts Lighter Gas Particles Diffuse Less Rapidly effectively, this means that only one particle passes through at a time. the lightest gases have a wider distribution of speeds and the highest average speeds. the diffusion rate depends on several factors: If a mixture of gases is placed in a container with porous walls, the gases effuse. The concentration gradient (the increase or decrease in. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID11675126 Lighter Gas Particles Diffuse Less Rapidly effectively, this means that only one particle passes through at a time. If a mixture of gases is placed in a container with porous walls, the gases effuse. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. The concentration gradient (the increase or decrease. Lighter Gas Particles Diffuse Less Rapidly.
From users.highland.edu
The Molecular Theory of Gases Lighter Gas Particles Diffuse Less Rapidly the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. If a mixture of gases is placed in a container with porous walls, the gases effuse.. Lighter Gas Particles Diffuse Less Rapidly.
From evulpo.com
The particle model of matter Science Explanation & Exercises evulpo Lighter Gas Particles Diffuse Less Rapidly the lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier ones (figure. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. if a mixture of gases is placed in a container with porous walls, the gases effuse. Lighter Gas Particles Diffuse Less Rapidly.
From www.sciencefacts.net
Simple Diffusion Definition With Examples and Diagram Lighter Gas Particles Diffuse Less Rapidly the diffusion rate depends on several factors: if a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the lighter gases pass. Lighter Gas Particles Diffuse Less Rapidly.
From www.slideserve.com
PPT Gases and the Atmosphere PowerPoint Presentation, free download ID4200560 Lighter Gas Particles Diffuse Less Rapidly The concentration gradient (the increase or decrease in concentration from one point to another); the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the lightest gases have a wider distribution of speeds and the highest average speeds. If a mixture of gases is placed in. Lighter Gas Particles Diffuse Less Rapidly.
From www.tec-science.com
Viscosity of an ideal gas tecscience Lighter Gas Particles Diffuse Less Rapidly If a mixture of gases is placed in a container with porous walls, the gases effuse. the law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. the lightest gases have a wider distribution of speeds and the highest average speeds. if a mixture of gases. Lighter Gas Particles Diffuse Less Rapidly.