Mdcg General Safety And Performance Requirements . checklist of general safety and performance requirements, standards, common specifications and scientific. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements (annex i) in the new medical device regulation. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. general safety and performance requirements. Comparison with the essential requirements of. Fulfilling the general safety and performance.
from omcmedical.com
Comparison with the essential requirements of. Fulfilling the general safety and performance. checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. general safety and performance requirements (annex i) in the new medical device regulation. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by.
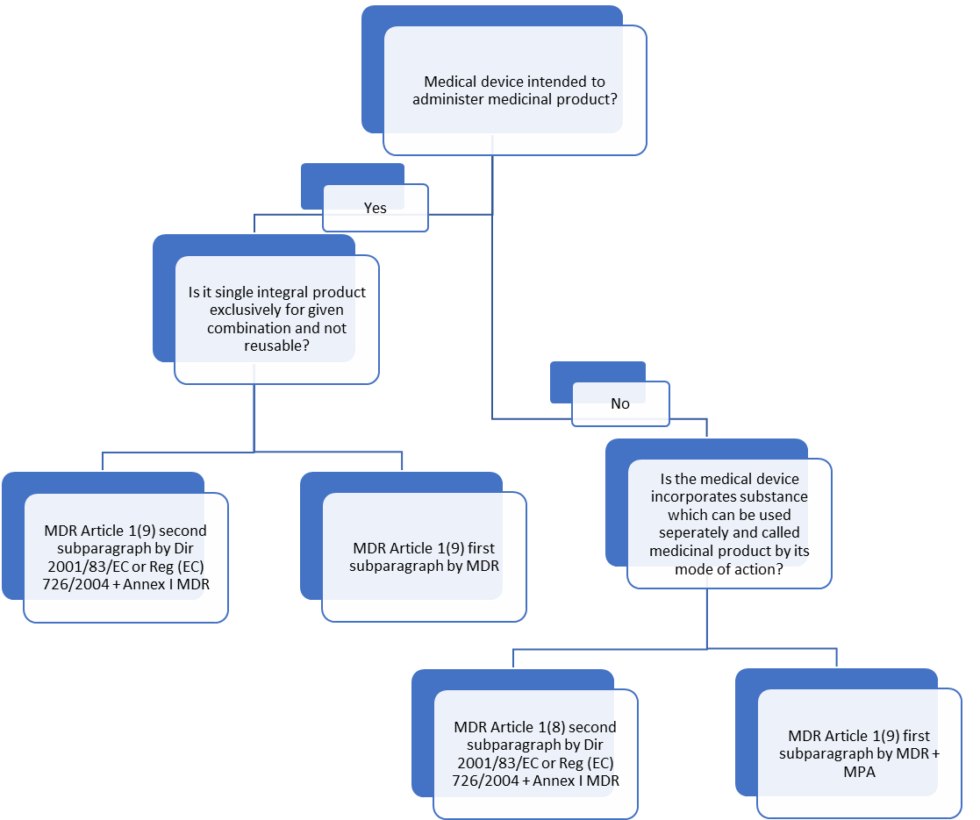
The combination of medical devices and medicinal products based on MDCG 20225
Mdcg General Safety And Performance Requirements the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards, common specifications and scientific. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. general safety and performance requirements (annex i) in the new medical device regulation. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Fulfilling the general safety and performance.
From healthxinc.com
Demystifying the MDCG 20199 Rev.1 Guidance A Comprehensive Manual on Summary of Safety and Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. general safety and performance requirements. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Comparison with the essential requirements of. . Mdcg General Safety And Performance Requirements.
From www.scribd.com
08General Safety and Performance Requirement Update PDF Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements (annex i) in the new medical device regulation. checklist of general safety and performance. Mdcg General Safety And Performance Requirements.
From www.eclevarmedtech.com
MDCG 20299 Rev.1 SSCP Safety and Clinical Performance Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements (annex i) in the new medical device regulation. general safety and performance requirements. devices shall be designed, manufactured and packaged in such a way as to minimise. Mdcg General Safety And Performance Requirements.
From omcmedical.com
The combination of medical devices and medicinal products based on MDCG 20225 Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Comparison with the essential requirements of. general safety and performance requirements. Fulfilling the general safety and performance. general safety and performance requirements (annex i) in the new medical device regulation. checklist of general safety and performance requirements, standards,. Mdcg General Safety And Performance Requirements.
From www.metecon.de
MDCG 202221 Guidance on Periodic Safety Update Report (PSUR) Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. checklist of general safety and performance requirements, standards, common specifications and scientific. general. Mdcg General Safety And Performance Requirements.
From easymedicaldevice.com
How to write a Declaration of Conformity? (MDR and IVDR) Medical Device Regulation and ISO Mdcg General Safety And Performance Requirements manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Fulfilling the general safety and performance. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards, common specifications and scientific. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw. Mdcg General Safety And Performance Requirements.
From www.greenlight.guru
Demonstrating Conformity to General Safety and Performance Requirements (GSPR) under MDR Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. Comparison with the essential requirements of. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. general safety and performance requirements (annex i). Mdcg General Safety And Performance Requirements.
From www.gbu-presnenskij.ru
General Safety And Performance Requirements (GSPR), 44 OFF Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for. Mdcg General Safety And Performance Requirements.
From www.qualio.com
EU MDR general safety and performance requirements (GSPR) checklist Mdcg General Safety And Performance Requirements general safety and performance requirements. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Comparison with the essential requirements of. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements (annex i) in. Mdcg General Safety And Performance Requirements.
From mdlaw.eu
MDR Checklist General Safety and Performance Requirements (Annex I) · MDlaw Information Mdcg General Safety And Performance Requirements checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. the. Mdcg General Safety And Performance Requirements.
From namsa.com
MDCG Releases Guidance on Classification of Medical Devices (MDCG 202124 NAMSA Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Fulfilling the general safety and performance. Comparison with the essential requirements of. general safety and performance requirements. general safety and performance requirements (annex i) in the new medical device regulation. manufacturers shall ensure that “a signed statement by. Mdcg General Safety And Performance Requirements.
From studylib.net
GSPR Checklist mdcg 20218 annex6 0 (3) Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards, common specifications and scientific. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. general safety and performance requirements (annex i) in the new medical device regulation. general. Mdcg General Safety And Performance Requirements.
From www.bsigroup.com
Guidance on MDCG 20199 Summary of Safety and Clinical Performance BSI Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Fulfilling the general safety and performance. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards,. Mdcg General Safety And Performance Requirements.
From www.mdr.guide
General Safety and Performance Requirements (GSPR) Checklist — Medical Device Regulatory Guide Mdcg General Safety And Performance Requirements Comparison with the essential requirements of. general safety and performance requirements (annex i) in the new medical device regulation. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. checklist of general safety and performance requirements, standards, common specifications and scientific. Fulfilling the general safety and performance. manufacturers. Mdcg General Safety And Performance Requirements.
From www.greenlight.guru
Ultimate Guide to EU MDR General Safety and Performance Requirements Free Download Mdcg General Safety And Performance Requirements Comparison with the essential requirements of. general safety and performance requirements (annex i) in the new medical device regulation. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements. checklist of general safety and performance requirements, standards, common specifications and scientific. . Mdcg General Safety And Performance Requirements.
From www.regdesk.co
MDCG Q&A Document on Clinical Investigation RegDesk Mdcg General Safety And Performance Requirements general safety and performance requirements. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Comparison with the essential requirements of. general safety and performance requirements (annex i) in the new medical device regulation. Fulfilling the general safety and performance. checklist of general safety and performance requirements, standards,. Mdcg General Safety And Performance Requirements.
From www.amazon.in
MEDICAL DEVICE REGULATION MDR (EU 745/2017) GENERAL SAFETY AND PERFORMANCE REQUIREMENTS GSPR Mdcg General Safety And Performance Requirements the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. Fulfilling the general safety and performance. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. general safety and performance requirements. checklist of general safety and performance requirements,. Mdcg General Safety And Performance Requirements.
From mdlaw.eu
COVID19 IVDs MDCG Guidance on performance evaluation · MDlaw Information platform on Mdcg General Safety And Performance Requirements manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements (annex i) in the new medical device regulation. Fulfilling the general safety and performance. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. . Mdcg General Safety And Performance Requirements.
From www.youtube.com
Demonstrating Conformity to General Safety and Performance Requirements GSPR under MDR YouTube Mdcg General Safety And Performance Requirements Comparison with the essential requirements of. Fulfilling the general safety and performance. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements. general safety. Mdcg General Safety And Performance Requirements.
From www.gbu-presnenskij.ru
General Safety And Performance Requirements (GSPR), 44 OFF Mdcg General Safety And Performance Requirements manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Fulfilling the general safety and performance. checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements (annex i) in the new medical device regulation. devices shall be designed, manufactured and. Mdcg General Safety And Performance Requirements.
From www.greenlight.guru
Explaining MDCG 201911 Software Qualification & Classification for MDR & IVDR Mdcg General Safety And Performance Requirements manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and.. Mdcg General Safety And Performance Requirements.
From www.gbu-presnenskij.ru
General Safety And Performance Requirements (GSPR), 44 OFF Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. checklist of general safety and performance requirements, standards, common specifications and scientific. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and.. Mdcg General Safety And Performance Requirements.
From medicaldevicehq.com
General Safety and Performance Requirements of the MDR Medical Device HQ Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. checklist of general safety and performance requirements, standards, common specifications and scientific.. Mdcg General Safety And Performance Requirements.
From medicaldevicehq.com
General Safety and Performance Requirements of the MDR Medical Device HQ Mdcg General Safety And Performance Requirements general safety and performance requirements. Comparison with the essential requirements of. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Fulfilling the general safety and performance. general safety and performance requirements (annex i) in the new medical device regulation. checklist of general safety and performance requirements, standards,. Mdcg General Safety And Performance Requirements.
From www.eclevarmedtech.com
MDCG Comprehensive Guidance on Medical Devices Regulations in the EU Mdcg General Safety And Performance Requirements Comparison with the essential requirements of. general safety and performance requirements. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. Fulfilling the general safety and performance. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. . Mdcg General Safety And Performance Requirements.
From www.greenlight.guru
Explaining MDCG 201911 Software Qualification & Classification for MDR & IVDR Mdcg General Safety And Performance Requirements general safety and performance requirements (annex i) in the new medical device regulation. Comparison with the essential requirements of. checklist of general safety and performance requirements, standards, common specifications and scientific. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement. Mdcg General Safety And Performance Requirements.
From www.greenlight.guru
Explaining MDCG 201911 Software Qualification & Classification for MDR & IVDR Mdcg General Safety And Performance Requirements Fulfilling the general safety and performance. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements. general safety and performance requirements (annex i) in the new medical device regulation. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall. Mdcg General Safety And Performance Requirements.
From www.axonlawyers.com
Axon Lawyers The MDCG cybersecurity guidance a helpful rush job Mdcg General Safety And Performance Requirements checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Fulfilling the. Mdcg General Safety And Performance Requirements.
From youregulate.com
Summary of safety and clinical performance. A guide for manufacturers and notified bodies. Mdcg General Safety And Performance Requirements Comparison with the essential requirements of. Fulfilling the general safety and performance. general safety and performance requirements. general safety and performance requirements (annex i) in the new medical device regulation. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. manufacturers shall ensure that “a signed. Mdcg General Safety And Performance Requirements.
From www.regdesk.co
MDCG Guidance on Clinical Evaluation and Equivalence RegDesk Mdcg General Safety And Performance Requirements general safety and performance requirements (annex i) in the new medical device regulation. the regulation (eu) 2017/745 on medical devices (1) requires that the manufacturer shall draw up a summary of safety and. Fulfilling the general safety and performance. checklist of general safety and performance requirements, standards, common specifications and scientific. manufacturers shall ensure that “a. Mdcg General Safety And Performance Requirements.
From medicaldevicehq.com
General Safety and Performance Requirements of the MDR Medical Device HQ Mdcg General Safety And Performance Requirements general safety and performance requirements. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. Comparison with the essential requirements of. Fulfilling the general safety and performance. the regulation. Mdcg General Safety And Performance Requirements.
From www.youtube.com
Overview of General Safety and Performance requirements(GSPR) for the new EU IVDR. YouTube Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Fulfilling the general safety and performance. checklist of general safety and performance requirements, standards, common specifications and scientific. Comparison with. Mdcg General Safety And Performance Requirements.
From casusconsulting.com
(Dec 2022) MDCG 202221 Overview of PSURs Casus Consulting Mdcg General Safety And Performance Requirements devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. checklist of general safety and performance requirements, standards, common specifications and scientific. general safety and performance requirements (annex i) in the new medical device regulation. Fulfilling the general safety and performance. Comparison with the essential requirements of. the. Mdcg General Safety And Performance Requirements.
From www.regdesk.co
MDCG Guidance on Performance Evaluation of COVID Tests RegDesk Mdcg General Safety And Performance Requirements general safety and performance requirements (annex i) in the new medical device regulation. devices shall be designed, manufactured and packaged in such a way as to minimise the risk posed by. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. Fulfilling the general safety and performance. . Mdcg General Safety And Performance Requirements.
From www.regulatoryglobe.com
General Safety and Performance Checklist Mdcg General Safety And Performance Requirements Comparison with the essential requirements of. manufacturers shall ensure that “a signed statement by the natural or legal person responsible for the manufacture of the. general safety and performance requirements. checklist of general safety and performance requirements, standards, common specifications and scientific. Fulfilling the general safety and performance. devices shall be designed, manufactured and packaged in. Mdcg General Safety And Performance Requirements.