What Are Carboxylic Acids Soluble In . carboxylic acids are similar in some respects to both ketones and alcohols. Carboxylic acids are typically soluble in water. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Like ketones, the carboxyl carbon is sp2. Palmitic acid [ch 3 (ch. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether.
from saylordotorg.github.io
carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. Like ketones, the carboxyl carbon is sp2. Palmitic acid [ch 3 (ch. carboxylic acids are similar in some respects to both ketones and alcohols. Carboxylic acids are typically soluble in water. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether.
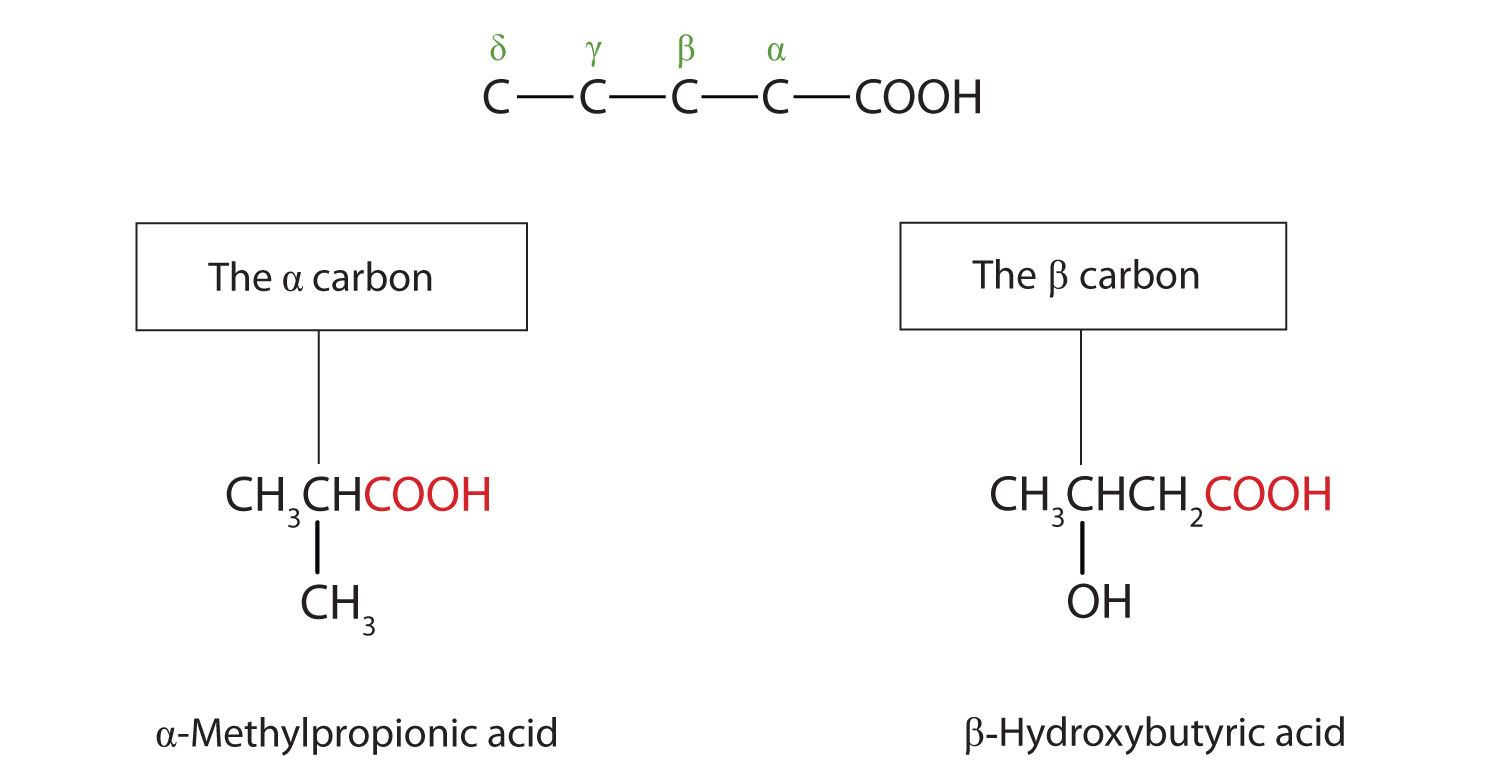
Carboxylic Acids Structures and Names
What Are Carboxylic Acids Soluble In the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. Carboxylic acids are typically soluble in water. Like ketones, the carboxyl carbon is sp2. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Palmitic acid [ch 3 (ch. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acids are similar in some respects to both ketones and alcohols. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water).
From www.chegg.com
Solved a. Are all carboxylic acids soluble in water? What Are Carboxylic Acids Soluble In Like ketones, the carboxyl carbon is sp2. carboxylic acids are similar in some respects to both ketones and alcohols. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. the solubility of carboxylic acids in water is similar. What Are Carboxylic Acids Soluble In.
From study.com
Carboxylic Acid Structure, Formula & Formation Lesson What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Their ability to form hydrogen bonds with water molecules allows them to dissolve. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT Chapter 20 Carboxylic Acids PowerPoint Presentation, free download ID294138 What Are Carboxylic Acids Soluble In Like ketones, the carboxyl carbon is sp2. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. carboxylic acid, any of a class of organic compounds in which a carbon (c). What Are Carboxylic Acids Soluble In.
From www.youtube.com
4 2 7 carboxylic acids forces, bp and solubility YouTube What Are Carboxylic Acids Soluble In Palmitic acid [ch 3 (ch. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded. What Are Carboxylic Acids Soluble In.
From www.sydney.edu.au
CARBOXYLIC ACIDS What Are Carboxylic Acids Soluble In the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT Functional Groups with the C = O Bond PowerPoint Presentation ID2961239 What Are Carboxylic Acids Soluble In hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Palmitic acid [ch 3 (ch. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. carboxylic acids are similar in some respects to both ketones and alcohols. Carboxylic acids are typically soluble. What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved Sort these carboxylic acids based on their solubility What Are Carboxylic Acids Soluble In Like ketones, the carboxyl carbon is sp2. Carboxylic acids are typically soluble in water. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Palmitic acid [ch 3 (ch. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the solubility of carboxylic acids. What Are Carboxylic Acids Soluble In.
From revise.im
Carboxylic Acids and Esters Revise.im What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. carboxylic acid, any of a class of organic compounds in. What Are Carboxylic Acids Soluble In.
From www.wikidoc.org
Carboxylic acid wikidoc What Are Carboxylic Acids Soluble In Palmitic acid [ch 3 (ch. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. carboxylic acids with more than six carbons are only. What Are Carboxylic Acids Soluble In.
From www.youtube.com
SK025 Solubility of Carboxylic Acids (Chapter 10; Part 3) YouTube What Are Carboxylic Acids Soluble In Carboxylic acids are typically soluble in water. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. Palmitic. What Are Carboxylic Acids Soluble In.
From www.numerade.com
SOLVED Part A Which carboxylic acid listed below the most water soluble? View Available Hint(s What Are Carboxylic Acids Soluble In carboxylic acids are similar in some respects to both ketones and alcohols. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Their ability to form hydrogen bonds with water molecules allows. What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved Sort these carboxylic acids based on their solubility What Are Carboxylic Acids Soluble In carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. Palmitic acid [ch 3 (ch. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether.. What Are Carboxylic Acids Soluble In.
From www.youtube.com
Carboxylic acids H bonds, BP, solubility Organic molecules meriSTEM YouTube What Are Carboxylic Acids Soluble In Carboxylic acids are typically soluble in water. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal. What Are Carboxylic Acids Soluble In.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols (Fischer Esterification What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acids are similar in some respects to both ketones and alcohols. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a.. What Are Carboxylic Acids Soluble In.
From chemistryguru.com.sg
Carboxylic Acid Reactions Organic Chem What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Carboxylic acids are typically soluble in water. carboxylic acids are similar in some respects to both ketones and alcohols. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. hexanoic acid [ch 3 (ch. What Are Carboxylic Acids Soluble In.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross out what does not apply What Are Carboxylic Acids Soluble In Like ketones, the carboxyl carbon is sp2. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Carboxylic acids are. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT Carboxylic Acids PowerPoint Presentation, free download ID2276706 What Are Carboxylic Acids Soluble In hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Carboxylic acids are typically soluble in water. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. . What Are Carboxylic Acids Soluble In.
From www.youtube.com
Chapter 7; Part 5; Carboxylic Acids; Solubility of Carboxylic Acids in Water YouTube What Are Carboxylic Acids Soluble In Like ketones, the carboxyl carbon is sp2. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. carboxylic acids are similar in some respects. What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved Sort these carboxylic acids based on their solubility What Are Carboxylic Acids Soluble In carboxylic acids are similar in some respects to both ketones and alcohols. Carboxylic acids are typically soluble in water. the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are.. What Are Carboxylic Acids Soluble In.
From www.doubtnut.com
[Telugu] Lower carboxylic acids are soluble in water due to What Are Carboxylic Acids Soluble In Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. Carboxylic acids are typically soluble in water. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). . What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved A. Carboxylic Acids and Their Salts Benzoic Acid What Are Carboxylic Acids Soluble In Carboxylic acids are typically soluble in water. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). carboxylic acids with more than six carbons are only slightly soluble in water, but the. What Are Carboxylic Acids Soluble In.
From saylordotorg.github.io
Physical Properties of Carboxylic Acids What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Carboxylic acids are typically soluble in water. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). carboxylic acids are similar in some respects to both ketones and alcohols. Like ketones, the. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT THEME Structure and chemical properties of carboxylic acids. Heterofunctional compounds What Are Carboxylic Acids Soluble In carboxylic acids are similar in some respects to both ketones and alcohols. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Carboxylic acids are typically soluble in water. Like ketones, the carboxyl carbon is sp2. Their ability to form hydrogen bonds with water molecules allows them to dissolve. What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved Sort these carboxylic acids based on their solubility What Are Carboxylic Acids Soluble In the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. Carboxylic acids are typically soluble in water. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. the solubility of carboxylic acids in water is similar to that of. What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved Sodium bicarbonate reacts with carboxylic acids to What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. Palmitic acid [ch 3 (ch. carboxylic acids are similar in some respects to both ketones and alcohols. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the carboxylic acids generally are soluble in such organic. What Are Carboxylic Acids Soluble In.
From saylordotorg.github.io
Carboxylic Acids Structures and Names What Are Carboxylic Acids Soluble In Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). carboxylic acid, any of a class of. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT Carboxylic Acids Reactions PowerPoint Presentation, free download ID5481924 What Are Carboxylic Acids Soluble In the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. carboxylic acids are similar in some respects to both ketones and alcohols. carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are. Carboxylic acids are typically soluble in water.. What Are Carboxylic Acids Soluble In.
From www.coursehero.com
[Solved] Why are some carboxylic acids soluble in water? Course Hero What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Their ability to form hydrogen bonds. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT Carboxylic Acids PowerPoint Presentation, free download ID744938 What Are Carboxylic Acids Soluble In Palmitic acid [ch 3 (ch. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Carboxylic acids are typically soluble in water. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. the solubility of carboxylic acids in water is similar to that of. What Are Carboxylic Acids Soluble In.
From www.doubtnut.com
[Odia] Carboxylic acids are more soluble in What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acids are similar in some respects to both ketones and alcohols. Palmitic acid [ch 3 (ch. Like ketones, the carboxyl carbon is sp2. Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. hexanoic acid. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT Chapter 10 Carboxylic Acids PowerPoint Presentation, free download ID1824139 What Are Carboxylic Acids Soluble In the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). Carboxylic acids are typically soluble in water. the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl. What Are Carboxylic Acids Soluble In.
From www.science-revision.co.uk
Carboxylic acids What Are Carboxylic Acids Soluble In the carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. hexanoic acid [ch 3 (ch 2) 4 cooh] is barely soluble in water (about 1.0 g/100 g of water). carboxylic acids are similar in some respects to both ketones and alcohols. carboxylic acid, any of a class of organic compounds. What Are Carboxylic Acids Soluble In.
From www.chegg.com
Solved This is the carboxylic functional group test. The What Are Carboxylic Acids Soluble In Like ketones, the carboxyl carbon is sp2. Carboxylic acids are typically soluble in water. carboxylic acid, any of a class of organic compounds in which a carbon (c) atom is bonded to an oxygen (o) atom by a double bond and to a. Palmitic acid [ch 3 (ch. the carboxylic acids generally are soluble in such organic solvents. What Are Carboxylic Acids Soluble In.
From www.slideserve.com
PPT THEME Structure and chemical properties of carboxylic acids. Heterofunctional compounds What Are Carboxylic Acids Soluble In Their ability to form hydrogen bonds with water molecules allows them to dissolve readily in. Palmitic acid [ch 3 (ch. the solubility of carboxylic acids in water is similar to that of alcohols, aldehydes, and ketones. carboxylic acids are similar in some respects to both ketones and alcohols. Carboxylic acids are typically soluble in water. hexanoic acid. What Are Carboxylic Acids Soluble In.