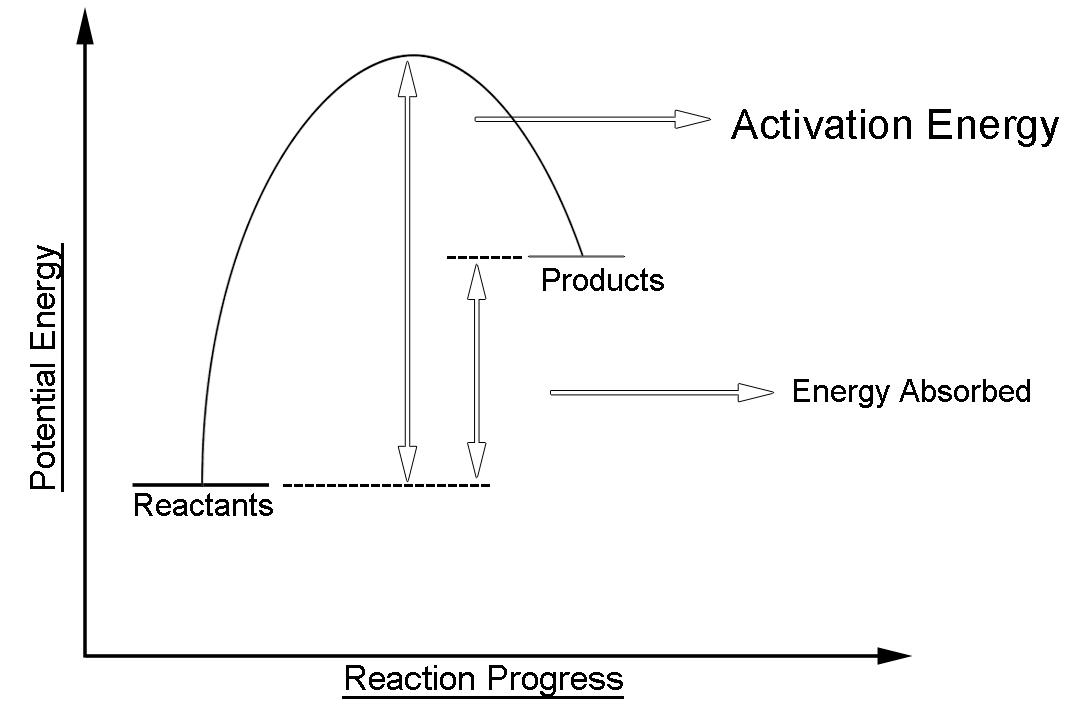
What Is Endothermic Reaction Simple Definition . These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. what is endothermic reaction? revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
from www.vedantu.com
what is endothermic reaction? an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
Explain Endothermic Reaction with examples?
What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. what is endothermic reaction? an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction that takes in energy from the surroundings.
From www.vedantu.com
Explain Endothermic Reaction with examples? What Is Endothermic Reaction Simple Definition endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. for an endothermic chemical reaction to proceed, the reactants must. What Is Endothermic Reaction Simple Definition.
From quizdbtranslunar.z21.web.core.windows.net
Examples Of Endothermic Reaction What Is Endothermic Reaction Simple Definition revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. for an. What Is Endothermic Reaction Simple Definition.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE What Is Endothermic Reaction Simple Definition an endothermic process is a chemical or physical process that absorbs heat from its surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. endothermic reactions are chemical reactions in which the reactants. What Is Endothermic Reaction Simple Definition.
From www.vrogue.co
Simple Endothermic Reaction Examples vrogue.co What Is Endothermic Reaction Simple Definition endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. what is endothermic reaction? an endothermic reaction is a chemical reaction that takes in energy from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. A. What Is Endothermic Reaction Simple Definition.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples What Is Endothermic Reaction Simple Definition A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. for an endothermic chemical reaction to. What Is Endothermic Reaction Simple Definition.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes What Is Endothermic Reaction Simple Definition for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. endothermic reactions are chemical reactions. What Is Endothermic Reaction Simple Definition.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples What Is Endothermic Reaction Simple Definition what is endothermic reaction? an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic reaction is a chemical reaction that takes in energy. What Is Endothermic Reaction Simple Definition.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science What Is Endothermic Reaction Simple Definition revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. These reactions lower. What Is Endothermic Reaction Simple Definition.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition, Properties, Examples What Is Endothermic Reaction Simple Definition for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an endothermic reaction is a. What Is Endothermic Reaction Simple Definition.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example What Is Endothermic Reaction Simple Definition A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. for an. What Is Endothermic Reaction Simple Definition.
From www.animalia-life.club
Endothermic Reaction Examples For Kids What Is Endothermic Reaction Simple Definition revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. . What Is Endothermic Reaction Simple Definition.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Presentation ID4835285 What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. endothermic reactions are chemical reactions in which the reactants absorb. What Is Endothermic Reaction Simple Definition.
From study.com
Endothermic Reaction Definition & Example Video & Lesson Transcript What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. A chemical reaction is said to be endothermic. What Is Endothermic Reaction Simple Definition.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic And Exothermic Reaction Diagram What Is Endothermic Reaction Simple Definition revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. what is endothermic reaction? an endothermic process is a chemical or physical process that absorbs heat from its surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from. What Is Endothermic Reaction Simple Definition.
From mavink.com
Endothermic And Exothermic Reactions What Is Endothermic Reaction Simple Definition These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. A chemical reaction is said to be endothermic when it absorbs. What Is Endothermic Reaction Simple Definition.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation What Is Endothermic Reaction Simple Definition endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. what is endothermic reaction? an endothermic reaction is a chemical reaction that takes in energy from the surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. for an endothermic chemical reaction to. What Is Endothermic Reaction Simple Definition.
From sciencenotes.org
Endothermic Reactions Definition and Examples What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. These reactions lower. What Is Endothermic Reaction Simple Definition.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry What Is Endothermic Reaction Simple Definition an endothermic process is a chemical or physical process that absorbs heat from its surroundings. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. what is endothermic reaction? A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. revise and understand what endothermic and exothermic reactions are. What Is Endothermic Reaction Simple Definition.
From www.aiophotoz.com
Endothermic Reaction Diagram Labeled Diagram Media Images and Photos finder What Is Endothermic Reaction Simple Definition what is endothermic reaction? endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. an endothermic process is a chemical or physical process that absorbs heat from its surroundings.. What Is Endothermic Reaction Simple Definition.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram What Is Endothermic Reaction Simple Definition endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. what is endothermic reaction? revise and understand what endothermic and exothermic reactions are and how the two reactions affect. What Is Endothermic Reaction Simple Definition.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. A chemical reaction is said to be endothermic when it. What Is Endothermic Reaction Simple Definition.
From www.youtube.com
Interpreting Exothermic and Endothermic Reaction Graphs A Simple Explanation of the Two What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. what is endothermic reaction? for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. These. What Is Endothermic Reaction Simple Definition.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing relation between reactant What Is Endothermic Reaction Simple Definition for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. an endothermic process is a chemical or physical process that. What Is Endothermic Reaction Simple Definition.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. what is endothermic reaction? endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical reaction to. What Is Endothermic Reaction Simple Definition.
From mavink.com
Diagram Of Endothermic Reaction What Is Endothermic Reaction Simple Definition These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. an endothermic reaction is a chemical change in which the system absorbs. What Is Endothermic Reaction Simple Definition.
From ask.modifiyegaraj.com
Endothermic Reaction Examples Science Struck Asking List What Is Endothermic Reaction Simple Definition A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. what is endothermic. What Is Endothermic Reaction Simple Definition.
From colouremployer8.gitlab.io
Supreme 4 Types Of Chemical Reactions Reactants And Products Cellular Respiration What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions in which the. What Is Endothermic Reaction Simple Definition.
From thechemistrynotes.com
Endothermic Reaction with Interesting Examples What Is Endothermic Reaction Simple Definition These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. what is endothermic reaction? revise and understand what endothermic. What Is Endothermic Reaction Simple Definition.
From classzonesalicetum.z14.web.core.windows.net
Identify Endothermic And Exothermic Reactions What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical reaction that takes in energy from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. what is endothermic reaction? endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. A. What Is Endothermic Reaction Simple Definition.
From www.thoughtco.com
Endothermic Reaction Examples What Is Endothermic Reaction Simple Definition These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical reaction to proceed, the reactants must absorb energy. What Is Endothermic Reaction Simple Definition.
From dxosvaiso.blob.core.windows.net
Endothermic Reaction Of Respiration at Calvin Petillo blog What Is Endothermic Reaction Simple Definition an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. an endothermic reaction. What Is Endothermic Reaction Simple Definition.
From www.youtube.com
simple endothermic reaction YouTube What Is Endothermic Reaction Simple Definition what is endothermic reaction? for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the. What Is Endothermic Reaction Simple Definition.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions What Is Endothermic Reaction Simple Definition what is endothermic reaction? revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic process is a chemical or physical process that absorbs heat from its surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from. What Is Endothermic Reaction Simple Definition.
From gamesmartz.com
Endothermic Reaction Easy to Understand What Is Endothermic Reaction Simple Definition for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. an. What Is Endothermic Reaction Simple Definition.
From www.youtube.com
Endothermic Reactions YouTube What Is Endothermic Reaction Simple Definition A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. an endothermic reaction is a chemical reaction that takes in energy from the surroundings. for an endothermic chemical reaction to proceed,. What Is Endothermic Reaction Simple Definition.