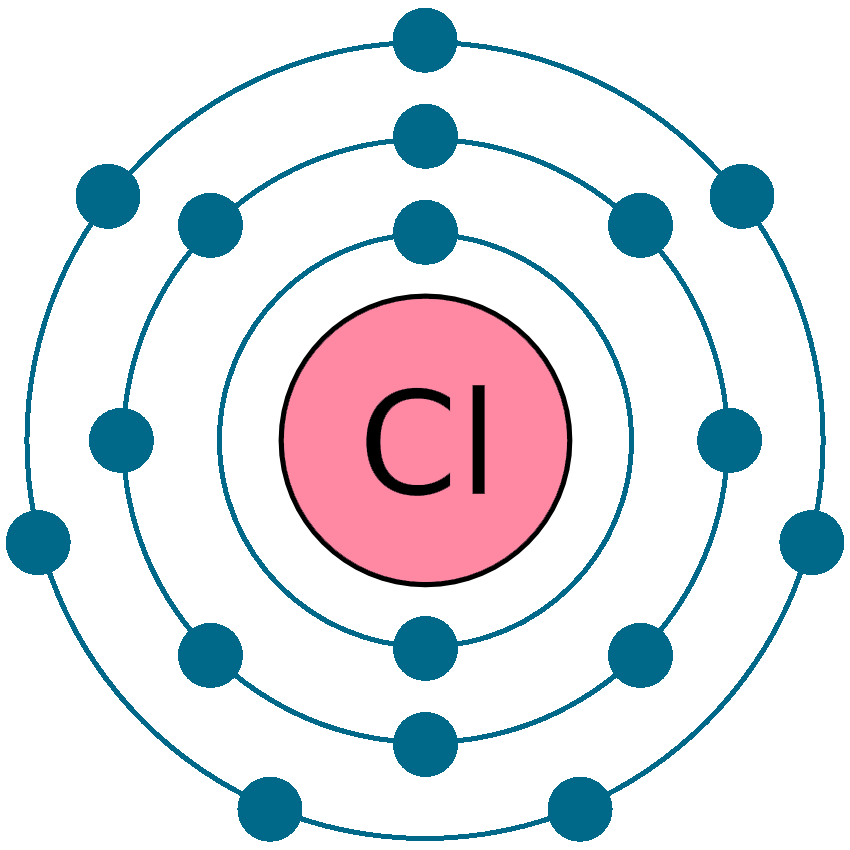
Chlorine Has 7 Electrons In The Valence Shell . group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. chlorine has 7 electrons in its valence shell. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). To meet the octet rule, it must either gain one electron or lose seven electrons.
from www.newtondesk.com
chlorine has 7 electrons in its valence shell. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. To meet the octet rule, it must either gain one electron or lose seven electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom.
Chlorine Cl (Element 17) of Periodic Table Newton Desk
Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). To meet the octet rule, it must either gain one electron or lose seven electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 electrons in its valence shell. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Has 7 Electrons In The Valence Shell the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. chlorine has 7 electrons in its valence shell. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. there are a total of seven electrons present in the valence shell/outermost shell of. Chlorine Has 7 Electrons In The Valence Shell.
From exovyidbs.blob.core.windows.net
How To Find Electron Shells In An Element at Jonathan Brown blog Chlorine Has 7 Electrons In The Valence Shell the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. group 17 elements, including fluorine and. Chlorine Has 7 Electrons In The Valence Shell.
From cebhjyoy.blob.core.windows.net
Chlorine Has 7 Valence Electrons. Why Would It Love To Gain Another Chlorine Has 7 Electrons In The Valence Shell chlorine has 7 electrons in its valence shell. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. To meet the octet rule, it must either gain one electron or lose seven electrons.. Chlorine Has 7 Electrons In The Valence Shell.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Has 7 Electrons In The Valence Shell an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. To meet the octet rule, it must either gain one electron or lose seven electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from. Chlorine Has 7 Electrons In The Valence Shell.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Has 7 Electrons In The Valence Shell To meet the octet rule, it must either gain one electron or lose seven electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. the #3s^2 3p^5# electrons are the outermost electrons, so. Chlorine Has 7 Electrons In The Valence Shell.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. To meet the octet rule, it must either. Chlorine Has 7 Electrons In The Valence Shell.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Has 7 Electrons In The Valence Shell To meet the octet rule, it must either gain one electron or lose seven electrons. chlorine has 7 electrons in its valence shell. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. chlorine has 7. Chlorine Has 7 Electrons In The Valence Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica Chlorine Has 7 Electrons In The Valence Shell chlorine has 7 electrons in its valence shell. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell. Chlorine Has 7 Electrons In The Valence Shell.
From circuitlistcarucage55.z22.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Has 7 Electrons In The Valence Shell group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 electrons in its valence. Chlorine Has 7 Electrons In The Valence Shell.
From www.pinterest.com
Valence Electrons• The electrons in the outer most electron shell are Chlorine Has 7 Electrons In The Valence Shell an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. the #3s^2 3p^5# electrons are. Chlorine Has 7 Electrons In The Valence Shell.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. To meet the octet rule, it must either gain. Chlorine Has 7 Electrons In The Valence Shell.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Has 7 Electrons In The Valence Shell an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. chlorine has 7 electrons in its valence shell. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them. Chlorine Has 7 Electrons In The Valence Shell.
From mavink.com
Periodic Table Valence Electrons Chlorine Has 7 Electrons In The Valence Shell chlorine has 7 electrons in its valence shell. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. To meet the octet rule, it must either gain one electron or lose seven electrons.. Chlorine Has 7 Electrons In The Valence Shell.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Chlorine Has 7 Electrons In The Valence Shell chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. To meet the octet rule, it must either gain one electron or lose seven electrons. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. an atom with a closed shell of valence. Chlorine Has 7 Electrons In The Valence Shell.
From mungfali.com
Periodic Table With Valence Electron Numbers Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). To meet the octet rule, it must either gain one electron or lose seven electrons. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. an atom with a closed shell. Chlorine Has 7 Electrons In The Valence Shell.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Chlorine Has 7 Electrons In The Valence Shell the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. To meet the octet rule, it must either gain one electron or lose seven electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. chlorine has 7 electrons in its valence shell. there. Chlorine Has 7 Electrons In The Valence Shell.
From utedzz.blogspot.com
Periodic Table Chlorine Bohr Model Periodic Table Timeline Chlorine Has 7 Electrons In The Valence Shell the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. group 17 elements, including. Chlorine Has 7 Electrons In The Valence Shell.
From www.britannica.com
halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). To meet the octet rule, it must either gain one electron or lose seven electrons. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. the #3s^2 3p^5# electrons are the. Chlorine Has 7 Electrons In The Valence Shell.
From www.expii.com
Valence Electrons — Definition & Importance Expii Chlorine Has 7 Electrons In The Valence Shell group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. the #3s^2 3p^5# electrons are. Chlorine Has 7 Electrons In The Valence Shell.
From mungfali.com
Valence Electron Shell Diagram Chlorine Has 7 Electrons In The Valence Shell To meet the octet rule, it must either gain one electron or lose seven electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. chlorine has 7 electrons. Chlorine Has 7 Electrons In The Valence Shell.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Has 7 Electrons In The Valence Shell chlorine has 7 electrons in its valence shell. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells,. Chlorine Has 7 Electrons In The Valence Shell.
From cebhjyoy.blob.core.windows.net
Chlorine Has 7 Valence Electrons. Why Would It Love To Gain Another Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. To meet the octet rule, it must either gain. Chlorine Has 7 Electrons In The Valence Shell.
From dxofcoqqf.blob.core.windows.net
How Many Valence Electrons Are There In Chlorine at Candace Kelly blog Chlorine Has 7 Electrons In The Valence Shell group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. chlorine has 7 electrons in its valence shell. there are a total of seven electrons present in the valence shell/outermost shell of chlorine. Chlorine Has 7 Electrons In The Valence Shell.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Has 7 Electrons In The Valence Shell the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 electrons in its valence shell. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to. Chlorine Has 7 Electrons In The Valence Shell.
From www.allaboutcircuits.com
Valence and Crystal Structure Solidstate Device Theory Electronics Chlorine Has 7 Electrons In The Valence Shell To meet the octet rule, it must either gain one electron or lose seven electrons. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from. Chlorine Has 7 Electrons In The Valence Shell.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Chlorine Has 7 Electrons In The Valence Shell group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). chlorine has 7 valence electrons because there. Chlorine Has 7 Electrons In The Valence Shell.
From ceypblhz.blob.core.windows.net
Valency Of Chlorine With Charge at Johnny Betancourt blog Chlorine Has 7 Electrons In The Valence Shell an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. To meet the octet rule, it must either gain one electron or lose seven electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). the #3s^2 3p^5# electrons are the outermost electrons,. Chlorine Has 7 Electrons In The Valence Shell.
From fwrnaissnschematic.z22.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Has 7 Electrons In The Valence Shell chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. chlorine has 7 electrons in its valence shell. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells,. Chlorine Has 7 Electrons In The Valence Shell.
From cebbfahb.blob.core.windows.net
Chlorine Atom Is Electrons at Jane Hayward blog Chlorine Has 7 Electrons In The Valence Shell To meet the octet rule, it must either gain one electron or lose seven electrons. there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms. Chlorine Has 7 Electrons In The Valence Shell.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Has 7 Electrons In The Valence Shell an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. there are a total. Chlorine Has 7 Electrons In The Valence Shell.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Has 7 Electrons In The Valence Shell the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. chlorine has 7 valence electrons because there are 7 electrons present in the outermost shell of the chlorine (cl) atom. chlorine has 7 electrons in its valence shell. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells,. Chlorine Has 7 Electrons In The Valence Shell.
From elchoroukhost.net
Chlorine Periodic Table Electrons Elcho Table Chlorine Has 7 Electrons In The Valence Shell there are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. To meet the octet rule, it must either gain one electron or lose seven electrons. chlorine has 7 electrons in its valence shell.. Chlorine Has 7 Electrons In The Valence Shell.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Has 7 Electrons In The Valence Shell group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. chlorine has 7 valence electrons because there are 7. Chlorine Has 7 Electrons In The Valence Shell.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Has 7 Electrons In The Valence Shell an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. To meet the octet rule, it. Chlorine Has 7 Electrons In The Valence Shell.
From sciencenotes.org
Chlorine Facts Chlorine Has 7 Electrons In The Valence Shell group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. an atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be. chlorine has 7 electrons in. Chlorine Has 7 Electrons In The Valence Shell.