Why Does Pressure Increase With Temperature . increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. In reality, most compression take. volume increases with increasing temperature or amount but decreases with increasing pressure. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. increased temperature would increase the energy of the molecules and the number of collisions would therefore. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. But if the same data is used and the graph extended to.
from www.teachoo.com
volume increases with increasing temperature or amount but decreases with increasing pressure. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. In reality, most compression take. increased temperature would increase the energy of the molecules and the number of collisions would therefore. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. But if the same data is used and the graph extended to. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure.
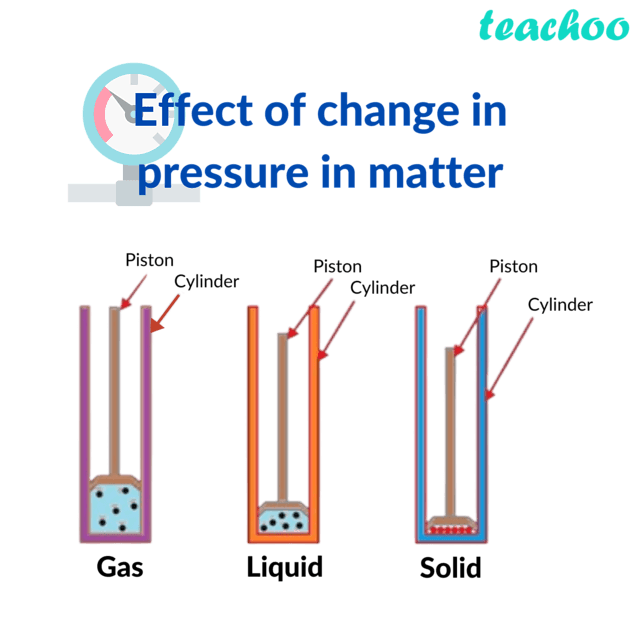
Changing Pressure to Change State of Matter Chemistry Teachoo
Why Does Pressure Increase With Temperature increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. volume increases with increasing temperature or amount but decreases with increasing pressure. increased temperature would increase the energy of the molecules and the number of collisions would therefore. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. But if the same data is used and the graph extended to. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two.
From slidetodoc.com
The relationship between temperature and volume How Volume Why Does Pressure Increase With Temperature But if the same data is used and the graph extended to. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. increased temperature would increase the energy of the molecules and the number of collisions would therefore. this graph tells us that as temperature increases, kinetic energy increases and. Why Does Pressure Increase With Temperature.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Why Does Pressure Increase With Temperature In reality, most compression take. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increased temperature would increase the energy of the molecules and the number of collisions would therefore. as the temperature increases, the pressure increases showing that pressure is directly proportional close. Why Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Aim What are factors of Air Pressure? PowerPoint Presentation Why Does Pressure Increase With Temperature if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. In reality, most compression take. this graph tells us that as temperature increases, kinetic energy increases and this. Why Does Pressure Increase With Temperature.
From gcsephysicsninja.com
11. Heating gas at a constant pressure Why Does Pressure Increase With Temperature But if the same data is used and the graph extended to. In reality, most compression take. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. volume increases with increasing temperature. Why Does Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Why Does Pressure Increase With Temperature In reality, most compression take. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. But if the same data is used and the graph extended to. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. volume. Why Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Why Does Pressure Increase With Temperature this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. But if the same data is used and the graph extended to. increased temperature would increase the energy of the molecules and the number of collisions would therefore. In reality, most compression take. as the temperature increases, the pressure. Why Does Pressure Increase With Temperature.
From techiescientist.com
Does Density Change With Temperature? Techiescientist Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. In reality, most compression take. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. volume increases with increasing temperature or amount but decreases with increasing pressure. increasing temperature increases the average. Why Does Pressure Increase With Temperature.
From dxoboaesr.blob.core.windows.net
Why Does Temp Increase With Pressure at Shane Bailey blog Why Does Pressure Increase With Temperature this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. volume increases with increasing temperature or amount but decreases with increasing pressure. In reality, most compression take. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more. Why Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Why Does Pressure Increase With Temperature as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. if you had a way to increase pressure with no volume change, then yes, temperature would. Why Does Pressure Increase With Temperature.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Why Does Pressure Increase With Temperature this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increased temperature would increase the energy of the molecules and the number of collisions would therefore. But. Why Does Pressure Increase With Temperature.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful. Why Does Pressure Increase With Temperature.
From circuitlistesoteric101.z22.web.core.windows.net
Simple Explanation Of Boyle's Law Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. volume increases with increasing temperature or amount but decreases with increasing pressure. But if the same data. Why Does Pressure Increase With Temperature.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Why Does Pressure Increase With Temperature increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. volume increases with increasing temperature or amount but decreases with increasing pressure. increased temperature would. Why Does Pressure Increase With Temperature.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. But if the same data is used and the graph extended to. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. this graph tells us that. Why Does Pressure Increase With Temperature.
From cekqhhub.blob.core.windows.net
Why Does Vapor Pressure Increase With Temperature at Jerrell Cox blog Why Does Pressure Increase With Temperature as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. if you had a way to increase pressure with no volume change, then yes, temperature would. Why Does Pressure Increase With Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Why Does Pressure Increase With Temperature if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. volume increases with increasing temperature or amount but decreases with increasing pressure. But if the same data is used and the graph extended to. In reality, most compression take. increased temperature would increase the energy. Why Does Pressure Increase With Temperature.
From quizrecentring.z21.web.core.windows.net
Why Does Temperature Increase With Altitude Why Does Pressure Increase With Temperature if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increased temperature would increase the energy of the molecules and the number of collisions would therefore. But if the same data is used and the graph extended to. In reality, most compression take. volume increases. Why Does Pressure Increase With Temperature.
From www.tec-science.com
Why do pressure and temperature increase during the compression of a Why Does Pressure Increase With Temperature In reality, most compression take. But if the same data is used and the graph extended to. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more. Why Does Pressure Increase With Temperature.
From dxoithjoo.blob.core.windows.net
Does Pressure Increase With Temperature at Mildred Blose blog Why Does Pressure Increase With Temperature But if the same data is used and the graph extended to. In reality, most compression take. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. volume increases with increasing temperature. Why Does Pressure Increase With Temperature.
From courses.lumenlearning.com
Phase Changes Physics Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. if you had a way. Why Does Pressure Increase With Temperature.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Why Does Pressure Increase With Temperature if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure.. Why Does Pressure Increase With Temperature.
From dxordmvmh.blob.core.windows.net
Why Does Temperature Increase With Pressure at Josephine Satchell blog Why Does Pressure Increase With Temperature volume increases with increasing temperature or amount but decreases with increasing pressure. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. In reality, most compression take. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases. Why Does Pressure Increase With Temperature.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. But if the same data is used and the graph extended to. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. as the temperature increases, the pressure increases. Why Does Pressure Increase With Temperature.
From aliyaiwal.blogspot.com
Pressure And Temperature Relationship Why Does Pressure Increase With Temperature as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. volume increases with increasing temperature or amount but decreases with increasing pressure. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increased temperature would increase the. Why Does Pressure Increase With Temperature.
From dxoithjoo.blob.core.windows.net
Does Pressure Increase With Temperature at Mildred Blose blog Why Does Pressure Increase With Temperature increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. But if the same data is used and the graph extended to. volume increases with increasing. Why Does Pressure Increase With Temperature.
From ceuulqzk.blob.core.windows.net
Why Does Pressure Change With Temperature at Brenda Marx blog Why Does Pressure Increase With Temperature as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. But if the same data is used and the graph extended to. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increased temperature would increase the energy. Why Does Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6307179 Why Does Pressure Increase With Temperature But if the same data is used and the graph extended to. increased temperature would increase the energy of the molecules and the number of collisions would therefore. volume increases with increasing temperature or amount but decreases with increasing pressure. In reality, most compression take. as the temperature increases, the pressure increases showing that pressure is directly. Why Does Pressure Increase With Temperature.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Why Does Pressure Increase With Temperature But if the same data is used and the graph extended to. In reality, most compression take. volume increases with increasing temperature or amount but decreases with increasing pressure. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increasing temperature increases the average kinetic. Why Does Pressure Increase With Temperature.
From dxoithjoo.blob.core.windows.net
Does Pressure Increase With Temperature at Mildred Blose blog Why Does Pressure Increase With Temperature In reality, most compression take. But if the same data is used and the graph extended to. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. increased. Why Does Pressure Increase With Temperature.
From www.slideserve.com
PPT 17 Equilibrium (AHL) PowerPoint Presentation, free download ID Why Does Pressure Increase With Temperature this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. In reality, most compression take. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. increased temperature would increase the energy of the molecules and the number of collisions would therefore. . Why Does Pressure Increase With Temperature.
From www.nagwa.com
Question Video Determining the Pressure Change of a Gas When Its Why Does Pressure Increase With Temperature increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions. this graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional. Why Does Pressure Increase With Temperature.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Why Does Pressure Increase With Temperature But if the same data is used and the graph extended to. increased temperature would increase the energy of the molecules and the number of collisions would therefore. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. as the temperature increases, the pressure increases. Why Does Pressure Increase With Temperature.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Why Does Pressure Increase With Temperature volume increases with increasing temperature or amount but decreases with increasing pressure. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. In reality, most compression take. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. But. Why Does Pressure Increase With Temperature.
From pediaa.com
Relationship Between Pressure and Temperature Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. But if the same data is used and the graph extended to. volume increases with increasing temperature or amount but decreases with increasing pressure. increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting. Why Does Pressure Increase With Temperature.
From www.slideshare.net
Air pressure Why Does Pressure Increase With Temperature increased temperature would increase the energy of the molecules and the number of collisions would therefore. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. as the temperature increases, the pressure increases showing that pressure is directly proportional close proportional when two. In reality,. Why Does Pressure Increase With Temperature.