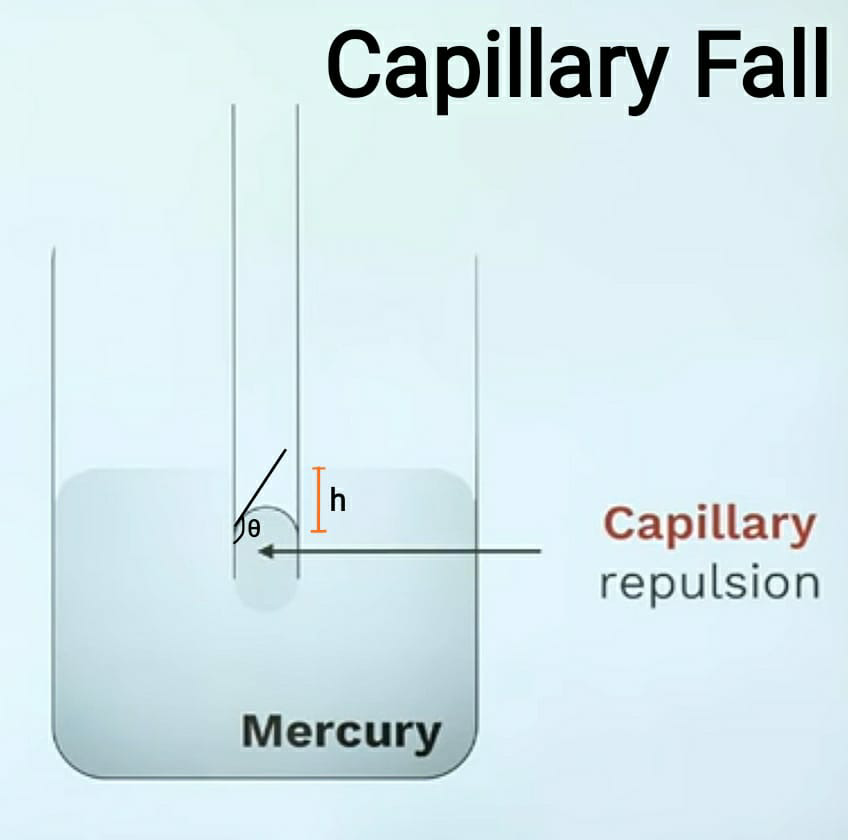
Surface Tension Capillarity Examples . Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension notes with solved examples. The surface tension depends on the two materials or mediums that it separates. The liquid interface is thus. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension is the energy required to increase. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length.
from civilquery.com
Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension is the energy required to increase. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. The liquid interface is thus. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. The surface tension depends on the two materials or mediums that it separates. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension notes with solved examples.
What is Surface Tension Definition, Examples and Tests Civil Query
Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. The liquid interface is thus. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. The surface tension depends on the two materials or mediums that it separates. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension is the energy required to increase. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension notes with solved examples.
From www.toppr.com
The correct formula to determine surface tension by capillary rise Surface Tension Capillarity Examples The liquid interface is thus. Surface tension notes with solved examples. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension is the energy required to increase. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. The surface tension depends on the two. Surface Tension Capillarity Examples.
From www.mechteaching.com
Capillarity Definition in Fluid Mechanics Surface Tension Capillarity Examples The liquid interface is thus. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy required to increase. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface. Surface Tension Capillarity Examples.
From www.youtube.com
(L7) What is Surface Tension Examples of Surface Tension Surface Tension Capillarity Examples Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces.. Surface Tension Capillarity Examples.
From www.expii.com
Cohesion and Adhesion — Definition & Overview Expii Surface Tension Capillarity Examples Surface tension is the energy required to increase. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension, capillary action, and viscosity are unique properties of. Surface Tension Capillarity Examples.
From www.aakash.ac.in
Capillarity Determination, Application & Examples AESL Surface Tension Capillarity Examples The surface tension depends on the two materials or mediums that it separates. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension is the energy required to increase. Liquids flow but adopt very stable shapes with surface fluctuations. Surface Tension Capillarity Examples.
From www.coursehero.com
[Solved] how will you explain surface tension and capillarity to an Surface Tension Capillarity Examples Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. The surface tension depends on the two materials or mediums that it separates. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Liquids flow but. Surface Tension Capillarity Examples.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. The liquid interface is thus. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension, capillary action, and viscosity are unique properties of liquids. Surface Tension Capillarity Examples.
From enggvacancy07.blogspot.com
Surface tension capillarity Effects Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. The surface tension depends on the two materials or. Surface Tension Capillarity Examples.
From www.slideserve.com
PPT Fluid Mechanics and Applications MECN 3110 PowerPoint Surface Tension Capillarity Examples Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. The surface tension depends on the two materials or mediums that it separates. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension, capillary action, and. Surface Tension Capillarity Examples.
From www.youtube.com
Concept of surface tension and capillarity YouTube Surface Tension Capillarity Examples The liquid interface is thus. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension notes with solved examples. Surface tension is the energy required to increase. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension \(\overline{\gamma}\) is. Surface Tension Capillarity Examples.
From gioksswwd.blob.core.windows.net
Surface Tension Model Equation at Jean Bernard blog Surface Tension Capillarity Examples Surface tension is the energy required to increase. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension notes with solved examples. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of. Surface Tension Capillarity Examples.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Capillarity Examples Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is the energy required to increase. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid.. Surface Tension Capillarity Examples.
From www.biolinscientific.com
Capillary action how contact angle and surface tension are related? Surface Tension Capillarity Examples Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is the energy required to increase. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension notes with solved examples. The liquid interface is. Surface Tension Capillarity Examples.
From www.youtube.com
Surface Tension of Water Capillary Action of Water Capillary Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension notes with solved examples. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension, capillary action, and. Surface Tension Capillarity Examples.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query Surface Tension Capillarity Examples Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension is the energy required to increase. The surface tension depends on the two materials or mediums that it separates. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Liquids flow but adopt very stable shapes with surface fluctuations limited. Surface Tension Capillarity Examples.
From www.youtube.com
Surface Tension, Capillarity YouTube Surface Tension Capillarity Examples Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension notes with solved examples. The surface tension depends on the two materials or mediums that it separates. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension \(\overline{\gamma}\) is defined to be the force f per. Surface Tension Capillarity Examples.
From scientificgamer.com
Capillary Action. The Scientific Gamer Surface Tension Capillarity Examples Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension notes with solved examples. The liquid interface is thus. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\). Surface Tension Capillarity Examples.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query Surface Tension Capillarity Examples Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is the energy required to increase. The liquid interface is thus. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions.. Surface Tension Capillarity Examples.
From enggvacancy07.blogspot.com
Surface tension capillarity Effects Surface Tension Capillarity Examples The liquid interface is thus. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length.. Surface Tension Capillarity Examples.
From www.youtube.com
Rise in liquid in Capillary Tube Surface Tension 12th Physics YouTube Surface Tension Capillarity Examples Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. The liquid interface is thus. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Calculate the diameter of a water droplet to attain. Surface Tension Capillarity Examples.
From byjus.com
What is the formula of capillarity? Surface Tension Capillarity Examples The surface tension depends on the two materials or mediums that it separates. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale.. Surface Tension Capillarity Examples.
From www.youtube.com
Surface tension and capillary rise YouTube Surface Tension Capillarity Examples Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is the energy required to increase. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. The surface tension depends on the. Surface Tension Capillarity Examples.
From www.aakash.ac.in
Capillarity Determination, Application & Examples AESL Surface Tension Capillarity Examples Surface tension is the energy required to increase. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. The liquid interface is thus. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension,. Surface Tension Capillarity Examples.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Surface Tension Capillarity Examples Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. The liquid interface. Surface Tension Capillarity Examples.
From www.quirkyscience.com
Capillary Action from the Forces of Adhesion and Cohesion Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit. Surface Tension Capillarity Examples.
From www.youtube.com
Surface Tension & Capillarity (Capillary Action) Experiment YouTube Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy required to increase. The liquid interface is thus. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension notes with solved examples. Surface tension \(\overline{\gamma}\) is defined to be the force. Surface Tension Capillarity Examples.
From www.animalia-life.club
What Is A Capillary Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. The liquid interface is thus. Surface tension \(\overline{\gamma}\) is defined to be the. Surface Tension Capillarity Examples.
From www.youtube.com
(1d) Surface Tension and Capillarity (Chapter Introduction) (Fluid Surface Tension Capillarity Examples Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is the energy required to increase.. Surface Tension Capillarity Examples.
From www.differencebetween.com
Difference Between Surface Tension and Capillary Action Compare the Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension notes with solved examples. Surface tension is the energy required to increase. Calculate. Surface Tension Capillarity Examples.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Surface Tension Capillarity Examples The surface tension depends on the two materials or mediums that it separates. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Surface tension is. Surface Tension Capillarity Examples.
From www.chegg.com
Solved Okay so which question so the F(upward shown above) Surface Tension Capillarity Examples Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. The surface tension depends on the two materials or mediums that it separates. Surface tension notes with solved examples. Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit. Surface Tension Capillarity Examples.
From sciencenotes.org
Capillary Action What It Is and How It Works Surface Tension Capillarity Examples Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension is the energy required to increase. The surface tension depends on the two materials or mediums that it separates. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Liquids flow but adopt very stable. Surface Tension Capillarity Examples.
From www.learncram.com
Capillarity in Physics Definition, Formula, Examples Surface Surface Tension Capillarity Examples Learn about formulae of surface energy, angle of contact, capillarity, applications, and intermolecular forces. Surface tension is the energy required to increase. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension notes with solved examples. Liquids flow but adopt very stable shapes with surface fluctuations limited to the. Surface Tension Capillarity Examples.
From www.youtube.com
SURFACE TENSION AND CAPILLARITY YouTube Surface Tension Capillarity Examples Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Liquids flow but adopt very stable shapes with surface fluctuations limited to the molecular scale. Surface tension \(\overline{\gamma}\) is defined to be the force f per unit length. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. The surface tension depends. Surface Tension Capillarity Examples.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension Capillarity Examples Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. The surface tension depends on the two materials or mediums that it separates. Calculate the diameter of a water droplet to attain pressure difference of 1000 [\ (n/m^2\)]. Surface tension is the energy required to increase. Surface tension notes with solved. Surface Tension Capillarity Examples.