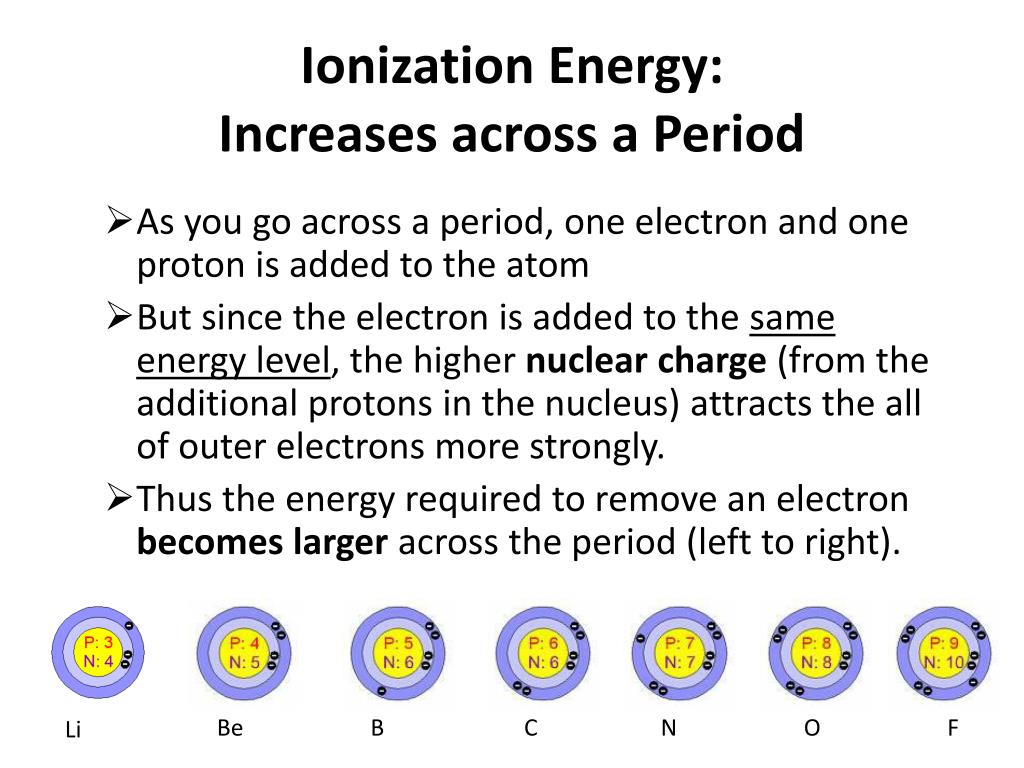
Why Does Ionization Energy Increase As You Remove Electrons . the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). Ionization energy exhibits periodicity on the periodic table. ionization energy is the energy needed to remove an electron from an atom or ion. Unlike atomic radii, we can and do. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second.
from www.slideserve.com
values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. ionization energy is the energy needed to remove an electron from an atom or ion. Unlike atomic radii, we can and do. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. Ionization energy exhibits periodicity on the periodic table.
PPT The Periodic Table PowerPoint Presentation, free download ID
Why Does Ionization Energy Increase As You Remove Electrons The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. Ionization energy exhibits periodicity on the periodic table. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. Unlike atomic radii, we can and do. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. ionization energy is the energy needed to remove an electron from an atom or ion. the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period.
From chemwiki.ucdavis.edu
5.6 Periodic Properties of the Elements Chemwiki Why Does Ionization Energy Increase As You Remove Electrons the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on. Why Does Ionization Energy Increase As You Remove Electrons.
From www.chem.fsu.edu
Electron Configurations Why Does Ionization Energy Increase As You Remove Electrons ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. the third ionisation energy shows a massive increase because it requires an electron to be removed from. Why Does Ionization Energy Increase As You Remove Electrons.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Why Does Ionization Energy Increase As You Remove Electrons the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. Ionization energy exhibits periodicity on the periodic table. ionization energy (the energy associated with forming a cation) decreases down. Why Does Ionization Energy Increase As You Remove Electrons.
From www.tes.com
AS Chemistry Ionisation Energy (Part 1) Teaching Resources Why Does Ionization Energy Increase As You Remove Electrons the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the ionization energy. Why Does Ionization Energy Increase As You Remove Electrons.
From byjus.com
Does NaorMghave higher ionization energy? Why Does Ionization Energy Increase As You Remove Electrons ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. ionization energy is the energy needed to remove an electron from an atom or ion. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). Unlike atomic radii, we can and do. . Why Does Ionization Energy Increase As You Remove Electrons.
From www.env.go.jp
Types of Ionizing Radiation [MOE] Why Does Ionization Energy Increase As You Remove Electrons the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the. Why Does Ionization Energy Increase As You Remove Electrons.
From www.aydemperakende.com.tr
What is Ionization? What Does Ionization Energy Mean? Aydem Perakende Why Does Ionization Energy Increase As You Remove Electrons ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. Ionization energy exhibits periodicity on the periodic table. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. values for the ionization energies of \(li\) and \(be\). Why Does Ionization Energy Increase As You Remove Electrons.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Why Does Ionization Energy Increase As You Remove Electrons the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). Ionization energy exhibits periodicity. Why Does Ionization Energy Increase As You Remove Electrons.
From pubchem.ncbi.nlm.nih.gov
Ionization Energy Periodic Table of Elements PubChem Why Does Ionization Energy Increase As You Remove Electrons The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. Ionization energy exhibits periodicity on the periodic table. the third ionisation energy shows a massive increase because it requires an electron to be. Why Does Ionization Energy Increase As You Remove Electrons.
From alevelchemistry.co.uk
Ionisation Energy ALevel Chemistry Revision Notes Why Does Ionization Energy Increase As You Remove Electrons ionization energy is the energy needed to remove an electron from an atom or ion. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. Unlike atomic radii, we can and do. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts. Why Does Ionization Energy Increase As You Remove Electrons.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Why Does Ionization Energy Increase As You Remove Electrons ionization energy is the energy needed to remove an electron from an atom or ion. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the.. Why Does Ionization Energy Increase As You Remove Electrons.
From www.ck12.org
Periodic Trends in Electronegativity CK12 Foundation Why Does Ionization Energy Increase As You Remove Electrons ionization energy is the energy needed to remove an electron from an atom or ion. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the nuclear. Why Does Ionization Energy Increase As You Remove Electrons.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Why Does Ionization Energy Increase As You Remove Electrons ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. Unlike. Why Does Ionization Energy Increase As You Remove Electrons.
From www.env.go.jp
Ionization due to Radiation [MOE] Why Does Ionization Energy Increase As You Remove Electrons Unlike atomic radii, we can and do. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the nuclear charge's pull on electrons has way more effect on the ionization energy. Why Does Ionization Energy Increase As You Remove Electrons.
From www.aydemperakende.com.tr
What is Ionization? What Does Ionization Energy Mean? Aydem Perakende Why Does Ionization Energy Increase As You Remove Electrons The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. ionization. Why Does Ionization Energy Increase As You Remove Electrons.
From socratic.org
Which element has a higher 3rd ionization energy, Al or Mg? Why? Socratic Why Does Ionization Energy Increase As You Remove Electrons values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. Ionization energy exhibits periodicity on the periodic table. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the nuclear charge's pull on electrons has way more. Why Does Ionization Energy Increase As You Remove Electrons.
From byjus.com
Calculate the ionization energy and separation energy for Li2+ ion if Why Does Ionization Energy Increase As You Remove Electrons ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. Unlike atomic radii, we can and do. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. values for the ionization energies of \(li\) and \(be\) listed. Why Does Ionization Energy Increase As You Remove Electrons.
From stock.adobe.com
Ionization energy (IE) Amount of energy required to remove the most Why Does Ionization Energy Increase As You Remove Electrons the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). values for the. Why Does Ionization Energy Increase As You Remove Electrons.
From www.pearson.com
Why does ionization energy increase regularly across the periodic Why Does Ionization Energy Increase As You Remove Electrons Unlike atomic radii, we can and do. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. ionization energy is the minimum energy required to remove an electron from. Why Does Ionization Energy Increase As You Remove Electrons.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Why Does Ionization Energy Increase As You Remove Electrons ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. the. Why Does Ionization Energy Increase As You Remove Electrons.
From phys.org
Understanding the periodic table through the lens of the volatile Group Why Does Ionization Energy Increase As You Remove Electrons the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. Unlike atomic radii, we. Why Does Ionization Energy Increase As You Remove Electrons.
From jackwestin.com
First And Second Ionization Energy The Periodic Table Variations Of Why Does Ionization Energy Increase As You Remove Electrons values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. ionization energy is the minimum energy required to remove an electron from an atom or ion in. Why Does Ionization Energy Increase As You Remove Electrons.
From inspiritvr.com
Periodic Trends Ionization Energy Study Guide Inspirit Learning Inc Why Does Ionization Energy Increase As You Remove Electrons the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. Unlike atomic radii, we can and do. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. ionization energy (the energy associated with forming a cation) decreases down a group and. Why Does Ionization Energy Increase As You Remove Electrons.
From slideplayer.com
WHAT HOW WHY Radius Electronegativity Ionization Energy. ppt download Why Does Ionization Energy Increase As You Remove Electrons Unlike atomic radii, we can and do. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a. Why Does Ionization Energy Increase As You Remove Electrons.
From www.tes.com
Ionisation Energy AS Level Teaching Resources Why Does Ionization Energy Increase As You Remove Electrons the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases. Why Does Ionization Energy Increase As You Remove Electrons.
From www.pearson.com
Why does ionization energy increase regularly across the periodic Why Does Ionization Energy Increase As You Remove Electrons The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). Ionization energy exhibits periodicity on the periodic table. the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\). Why Does Ionization Energy Increase As You Remove Electrons.
From www.pearson.com
Why does ionization energy increase regularly across the periodic Why Does Ionization Energy Increase As You Remove Electrons the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). Ionization energy exhibits periodicity on the periodic table. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across. Why Does Ionization Energy Increase As You Remove Electrons.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Why Does Ionization Energy Increase As You Remove Electrons ionization energy is the energy needed to remove an electron from an atom or ion. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. the nuclear. Why Does Ionization Energy Increase As You Remove Electrons.
From www.clutchprep.com
Periodic Trend Successive Ionization Energies Chemistry Video Why Does Ionization Energy Increase As You Remove Electrons the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. ionization energy is the energy needed to remove an electron from an atom or ion. ionization energy (the energy. Why Does Ionization Energy Increase As You Remove Electrons.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Why Does Ionization Energy Increase As You Remove Electrons the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. Unlike atomic radii, we can and do. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because. Why Does Ionization Energy Increase As You Remove Electrons.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Why Does Ionization Energy Increase As You Remove Electrons Ionization energy exhibits periodicity on the periodic table. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts (ev). ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. the nuclear charge's pull on electrons has way more effect on the ionization energy than. Why Does Ionization Energy Increase As You Remove Electrons.
From study.com
Ionization Energy Definition, Trends & Factors Video & Lesson Why Does Ionization Energy Increase As You Remove Electrons Ionization energy exhibits periodicity on the periodic table. Unlike atomic radii, we can and do. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. values for the ionization energies of. Why Does Ionization Energy Increase As You Remove Electrons.
From socratic.org
What are the periodic trends for atomic radii, ionization energy, and Why Does Ionization Energy Increase As You Remove Electrons the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element.. Why Does Ionization Energy Increase As You Remove Electrons.
From www.tes.com
Periodic Trends First Ionisation Energy OCR A Level Teaching Resources Why Does Ionization Energy Increase As You Remove Electrons the ionization energy required for removal of electrons increases progressively as the atom loses electrons, because the positive charge on the. Ionization energy exhibits periodicity on the periodic table. values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. The most common units of ionization energy are. Why Does Ionization Energy Increase As You Remove Electrons.
From pressbooks.online.ucf.edu
4.4 Ionization energy and Electron Affinity Chemistry Fundamentals Why Does Ionization Energy Increase As You Remove Electrons the third ionisation energy shows a massive increase because it requires an electron to be removed from magnesium’s second. the nuclear charge's pull on electrons has way more effect on the ionization energy than the gradual. Unlike atomic radii, we can and do. The most common units of ionization energy are kilojoules per mole (kj/m) or electron volts. Why Does Ionization Energy Increase As You Remove Electrons.