Discussion Of Titration Hcl With Naoh . One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl (hydrochloric. Volume measurements play a key role in titration. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Relating titrations to stoichiometry and limiting reagent problems. The initial volume and final volume of all three trails were subtracted to find each amount of titrant. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.
from www.learnsci.com
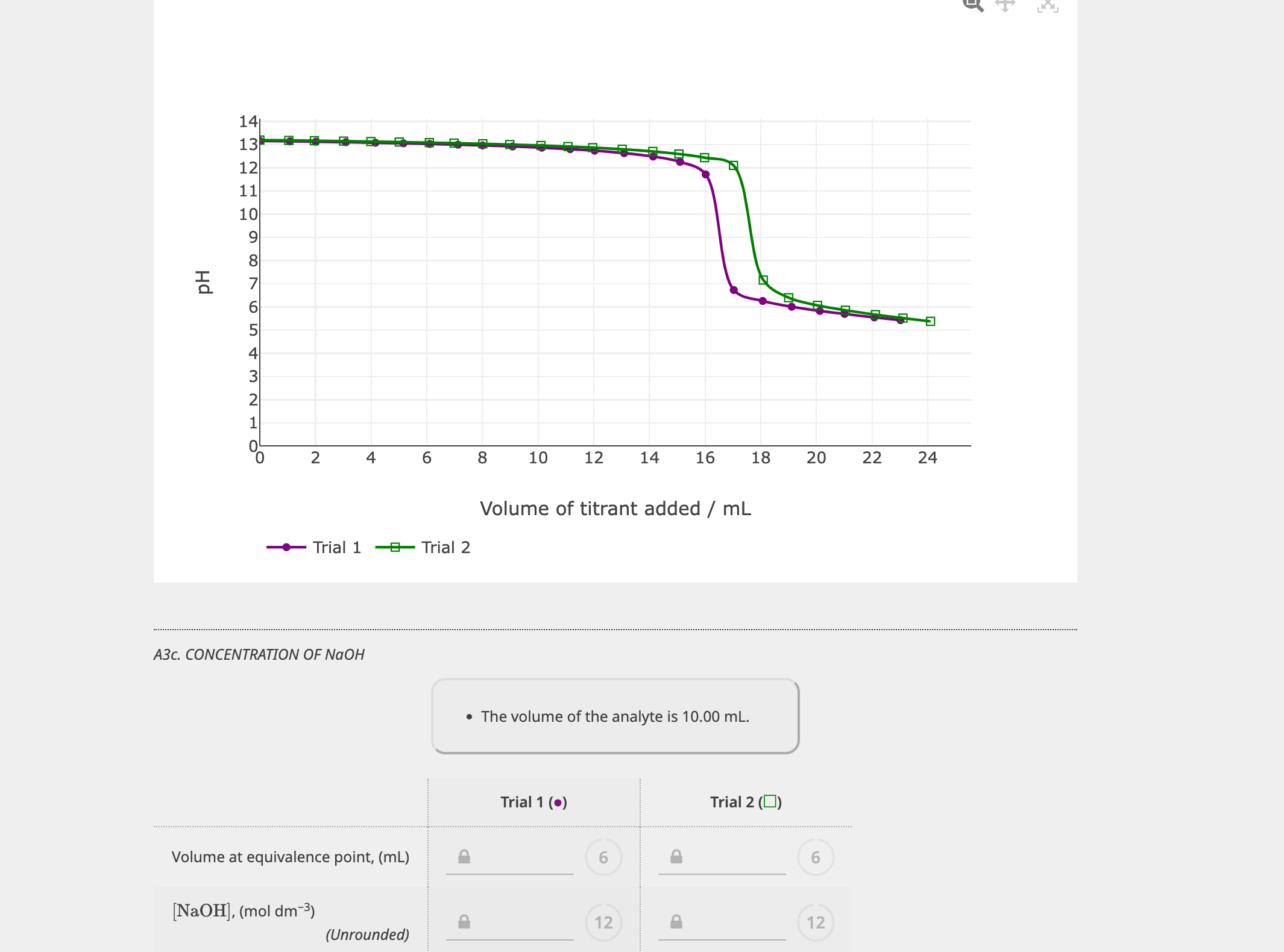
Volume measurements play a key role in titration. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. The initial volume and final volume of all three trails were subtracted to find each amount of titrant. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In equation 1, the acid is hcl (hydrochloric. Includes kit list and safety instructions. Relating titrations to stoichiometry and limiting reagent problems.
LearnSci Smart Worksheet Determine Concentration of HCl by Titration
Discussion Of Titration Hcl With Naoh Relating titrations to stoichiometry and limiting reagent problems. Volume measurements play a key role in titration. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. In equation 1, the acid is hcl (hydrochloric. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. The initial volume and final volume of all three trails were subtracted to find each amount of titrant. Relating titrations to stoichiometry and limiting reagent problems. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Relating titrations to stoichiometry and limiting reagent problems. Includes kit list and safety instructions. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\). Discussion Of Titration Hcl With Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Discussion Of Titration Hcl With Naoh The initial volume and final volume of all three trails were subtracted to find each amount of titrant. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Use this class practical to explore titration, producing the salt sodium. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Volume measurements play a key role in titration. Includes kit list and safety instructions. In equation 1, the acid is hcl (hydrochloric. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Relating titrations to stoichiometry and limiting reagent problems. In equation 1, the acid is hcl (hydrochloric. Volume measurements play a. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Includes kit list and safety instructions. Volume measurements play a key role in titration. Relating titrations to stoichiometry and limiting reagent problems. The initial volume and final volume of. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Includes kit list and safety instructions. Volume measurements play a key role in titration. Relating titrations to stoichiometry and limiting reagent problems. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume. Discussion Of Titration Hcl With Naoh.
From www.studypool.com
SOLUTION Titration of hydrochloric acid against sodium hydroxide Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl (hydrochloric. Volume measurements play a key role in titration. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Includes kit list and. Discussion Of Titration Hcl With Naoh.
From www.numerade.com
SOLVED When examining the titrations of hydrochloric acid and acetic Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl (hydrochloric. For the titration of a monoprotic strong acid (hcl) with a monobasic. Discussion Of Titration Hcl With Naoh.
From mungfali.com
Titration Curve HCl And NaOH Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Relating titrations to stoichiometry and limiting reagent problems. Includes kit list and safety instructions. The initial volume and final volume. Discussion Of Titration Hcl With Naoh.
From klaimjxhd.blob.core.windows.net
Titration Between Hcl And Naoh at Tamika Spear blog Discussion Of Titration Hcl With Naoh Volume measurements play a key role in titration. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Use this class practical to explore titration, producing the salt sodium chloride. Discussion Of Titration Hcl With Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Discussion Of Titration Hcl With Naoh The initial volume and final volume of all three trails were subtracted to find each amount of titrant. Volume measurements play a key role in titration. In equation 1, the acid is hcl (hydrochloric. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. Discussion Of Titration Hcl With Naoh.
From studylib.net
3. Titration NaOH and HCl concentration of an alkali Discussion Of Titration Hcl With Naoh Relating titrations to stoichiometry and limiting reagent problems. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The initial volume and final volume of all three trails were subtracted to find each amount of titrant. Volume measurements play a key role in titration. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the. Discussion Of Titration Hcl With Naoh.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Discussion Of Titration Hcl With Naoh In equation 1, the acid is hcl (hydrochloric. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Relating titrations to stoichiometry and limiting reagent problems. The initial volume and final volume of all three trails were subtracted to find each amount of titrant. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Volume measurements play a key role in titration. In equation. Discussion Of Titration Hcl With Naoh.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Discussion Of Titration Hcl With Naoh In equation 1, the acid is hcl (hydrochloric. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Volume measurements play a key role in titration. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. One type of titration uses a neutralization reaction,. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Includes kit list and safety instructions. In equation 1, the acid is hcl (hydrochloric. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Relating titrations to stoichiometry and limiting. Discussion Of Titration Hcl With Naoh.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Discussion Of Titration Hcl With Naoh Volume measurements play a key role in titration. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl (hydrochloric. Includes kit list and safety instructions. Relating titrations to stoichiometry and limiting reagent problems. For the titration of a monoprotic strong acid. Discussion Of Titration Hcl With Naoh.
From www.studypool.com
SOLUTION Titration with hcl and naoh Studypool Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Volume measurements play a key role in titration. Relating titrations to stoichiometry and limiting reagent problems. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Volume measurements play a key role in titration. Includes kit list and safety instructions. In equation 1, the acid is hcl (hydrochloric. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. For the. Discussion Of Titration Hcl With Naoh.
From studykaki.com
Describe a method of preparing sodium chloride from the reaction of Discussion Of Titration Hcl With Naoh For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. In equation 1, the acid is hcl (hydrochloric. Relating titrations to stoichiometry and limiting reagent problems. The initial volume and final volume of all three trails were subtracted to. Discussion Of Titration Hcl With Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In equation 1, the acid is hcl (hydrochloric. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. The initial volume and final volume of all three trails were subtracted to find each amount. Discussion Of Titration Hcl With Naoh.
From studymoose.com
Sodium Hydroxide & Hydrochloric Acid Titration Comparing Methods Discussion Of Titration Hcl With Naoh Volume measurements play a key role in titration. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Relating titrations to stoichiometry and limiting reagent problems. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In equation 1, the acid is hcl (hydrochloric.. Discussion Of Titration Hcl With Naoh.
From www.studypool.com
SOLUTION Rvs chemistry 11 hydrochloric acid and sodium hydroxide Discussion Of Titration Hcl With Naoh Volume measurements play a key role in titration. Includes kit list and safety instructions. Relating titrations to stoichiometry and limiting reagent problems. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The initial volume and final volume of all three trails were subtracted to find each amount. Discussion Of Titration Hcl With Naoh.
From klaimjxhd.blob.core.windows.net
Titration Between Hcl And Naoh at Tamika Spear blog Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Use this class practical to explore titration,. Discussion Of Titration Hcl With Naoh.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Volume measurements play a key role in titration. Relating titrations to stoichiometry and limiting reagent problems. The initial volume and final volume of all three trails were subtracted to find each amount of titrant. For the titration of a monoprotic strong acid. Discussion Of Titration Hcl With Naoh.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Discussion Of Titration Hcl With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. The initial volume and final volume of all three trails were subtracted to find each amount of titrant. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Volume measurements. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Volume measurements play a key role in titration. Relating titrations to stoichiometry and limiting reagent problems. The initial. Discussion Of Titration Hcl With Naoh.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Discussion Of Titration Hcl With Naoh The initial volume and final volume of all three trails were subtracted to find each amount of titrant. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. For the. Discussion Of Titration Hcl With Naoh.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Discussion Of Titration Hcl With Naoh In equation 1, the acid is hcl (hydrochloric. Includes kit list and safety instructions. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Volume measurements play a key role. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The initial volume and final volume of all three trails were subtracted to find each amount of titrant. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume. Discussion Of Titration Hcl With Naoh.
From mungfali.com
HCl NaOH Titration Discussion Of Titration Hcl With Naoh For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Volume measurements play a key role in titration. In equation 1, the acid is hcl (hydrochloric. Relating titrations to stoichiometry and limiting reagent problems. One type of titration uses. Discussion Of Titration Hcl With Naoh.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Discussion Of Titration Hcl With Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Relating titrations to stoichiometry and limiting reagent problems. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the. Discussion Of Titration Hcl With Naoh.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Discussion Of Titration Hcl With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Includes kit list and safety instructions. The initial volume and final volume of all three trails were subtracted to find each amount of titrant.. Discussion Of Titration Hcl With Naoh.
From loehmowzt.blob.core.windows.net
Indicator Used In Titration Of Naoh And Hcl at Dan Carlson blog Discussion Of Titration Hcl With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. For the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the equivalence point from the following. Volume measurements play a key role in titration. Use this class. Discussion Of Titration Hcl With Naoh.