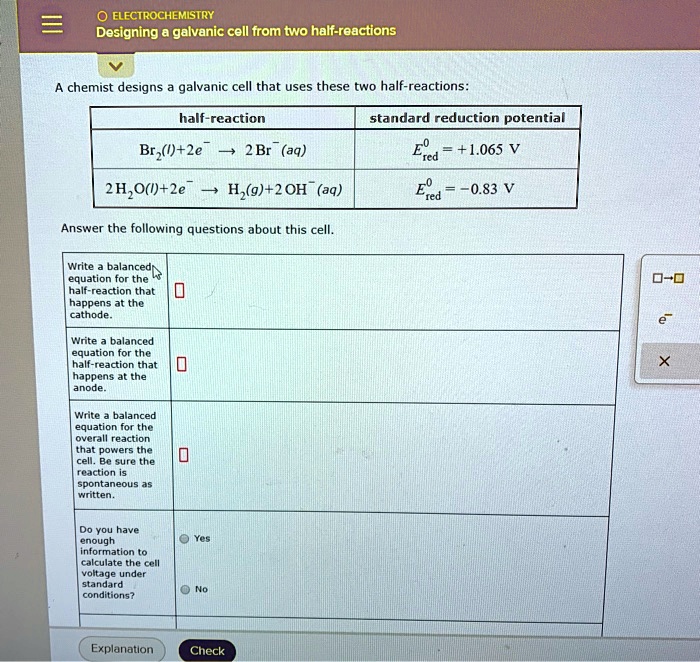
Designing A Galvanic Cell From Two Half-Reactions . One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. How to predict the voltage and overall reaction of a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3.
from www.numerade.com
The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons.
SOLVED ELECTROCHEMISTRY Designing a Galvanic Cell from Two Half
Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. How to predict the voltage and overall reaction of a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons.
From www.numerade.com
ELECTROCHEMISTRY Designing galvanic cell from two halfreactions Designing A Galvanic Cell From Two Half-Reactions One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper,. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED Designing a galvanic cell from two halfreactions chemist Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED Elecirochcmistry galvanic cell from Tno halfrcactions Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions A Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper,. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions A Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing a Galvanic Cell from Two Half Designing A Galvanic Cell From Two Half-Reactions One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From chemistrynotes.com
Redox Reaction Examples The Galvanic Cell Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved O ELECTROCHEMISTRY Designing a galvanic cell from two Designing A Galvanic Cell From Two Half-Reactions How to predict the voltage and overall reaction of a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVEDADVANCED MATERIAL Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved O ELECTROCHEMISTRY Designing a galvanic cell from two Designing A Galvanic Cell From Two Half-Reactions One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From www.youtube.com
ALEKS Designing a galvanic cell from two halfreactions YouTube Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions A Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions A Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons.. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions A Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVEDA chemist designs galvanic cell that uses these two half Designing A Galvanic Cell From Two Half-Reactions How to predict the voltage and overall reaction of a galvanic cell. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions.. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved O ELECTROCHEMISTRY Designing a galvanic cell from two Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains. Designing A Galvanic Cell From Two Half-Reactions.
From www.coursehero.com
[Solved] ELECTROCHEMISTRY Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions.. Designing A Galvanic Cell From Two Half-Reactions.
From www.coursehero.com
[Solved] ELECTROCHEMISTRY Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell.. Designing A Galvanic Cell From Two Half-Reactions.
From www.answersarena.com
[Solved] Designing a galvanic cell from two halfreact Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based. Designing A Galvanic Cell From Two Half-Reactions.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Designing A Galvanic Cell From Two Half-Reactions One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. See examples of galvanic cells based on spontaneous redox reactions between copper,. Designing A Galvanic Cell From Two Half-Reactions.
From www.coursehero.com
[Solved] ELECTROCHEMISTRY Designing a galvanic cell from two half Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions Designing A Galvanic Cell From Two Half-Reactions How to predict the voltage and overall reaction of a galvanic cell. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED EecirocheMSR Designing galvanic cell from two halfreactions A Designing A Galvanic Cell From Two Half-Reactions A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved O ELECTROCHEMISTRY Designing a galvanic cell from two Designing A Galvanic Cell From Two Half-Reactions See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. How to predict the voltage and overall reaction of a galvanic cell. The redox reaction is faradic reaction,. Designing A Galvanic Cell From Two Half-Reactions.
From www.numerade.com
SOLVED Title Designing a Galvanic Cell from Two HalfReactions A Designing A Galvanic Cell From Two Half-Reactions How to predict the voltage and overall reaction of a galvanic cell. See examples of galvanic cells based on spontaneous redox reactions between copper, silver,. The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure. Designing A Galvanic Cell From Two Half-Reactions.
From www.chegg.com
Solved Designing a galvanic cell from two halfreactions Designing A Galvanic Cell From Two Half-Reactions The redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. How to predict the voltage and overall reaction of a galvanic cell.. Designing A Galvanic Cell From Two Half-Reactions.