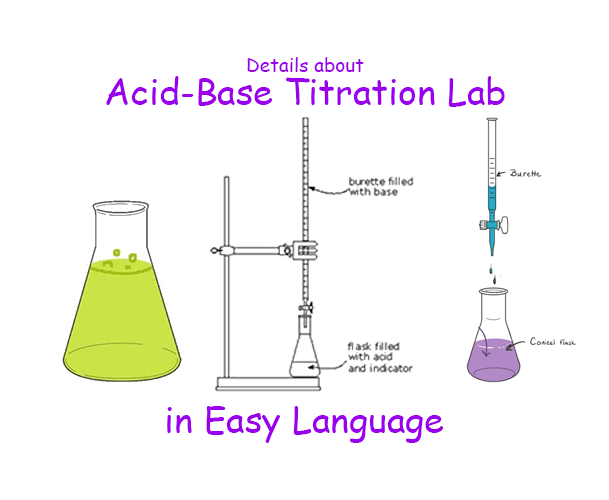
Titrimetric Analysis Lab . titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in this document, we will examine some of the specifics of titrimetric analysis: in chemistry, titrimetric methods are broadly classified into three types:
from gioldvmyt.blob.core.windows.net
in chemistry, titrimetric methods are broadly classified into three types: in this document, we will examine some of the specifics of titrimetric analysis: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that.
Titration Chemical Method at Karl Twiggs blog
Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in chemistry, titrimetric methods are broadly classified into three types: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in this document, we will examine some of the specifics of titrimetric analysis: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s.
From www.shutterstock.com
Chemical Laboratory Analysis Titration Stock Photo 433965310 Shutterstock Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in chemistry, titrimetric methods are broadly classified into three types: titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s). Titrimetric Analysis Lab.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Titrimetric Analysis Lab in this document, we will examine some of the specifics of titrimetric analysis: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in. Titrimetric Analysis Lab.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titrimetric Analysis Lab in this document, we will examine some of the specifics of titrimetric analysis: in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant. Titrimetric Analysis Lab.
From www.youtube.com
Lab 8 Volumetric Analysis, An AcidBase Titration YouTube Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of. Titrimetric Analysis Lab.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which. Titrimetric Analysis Lab.
From www.youtube.com
Titrimetric Methods of Analysis Analytical Chemistry YouTube Titrimetric Analysis Lab in this document, we will examine some of the specifics of titrimetric analysis: in chemistry, titrimetric methods are broadly classified into three types: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q). Titrimetric Analysis Lab.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in chemistry, titrimetric methods are broadly classified into three types: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in this document, we. Titrimetric Analysis Lab.
From theedge.com.hk
Chemistry How To Titration The Edge Titrimetric Analysis Lab in this document, we will examine some of the specifics of titrimetric analysis: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q). Titrimetric Analysis Lab.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in this document, we will examine some of the specifics of titrimetric analysis: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. . Titrimetric Analysis Lab.
From exyxqrdzm.blob.core.windows.net
Chemistry Titration Indicators at Richard Veit blog Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has. Titrimetric Analysis Lab.
From www.slideserve.com
PPT Titrimetry PowerPoint Presentation, free download ID2261229 Titrimetric Analysis Lab in chemistry, titrimetric methods are broadly classified into three types: in this document, we will examine some of the specifics of titrimetric analysis: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. titrimetric analysis involves determination of. Titrimetric Analysis Lab.
From www.studocu.com
Lab Back Titration N/A Topic Titrimetric Analysis Title Back Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in this document, we will examine some of the specifics of titrimetric analysis: in chemistry, titrimetric methods are broadly classified into three types: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative. Titrimetric Analysis Lab.
From pharmacyscope.com
How is titrimetric analysis calculated? Pharmacy Scope Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in this document, we will examine some of the specifics of titrimetric analysis: . Titrimetric Analysis Lab.
From sciencephotogallery.com
Titration Experiment by Titrimetric Analysis Lab in chemistry, titrimetric methods are broadly classified into three types: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. titration (also known as titrimetry[1] and volumetric analysis) is a common. Titrimetric Analysis Lab.
From www.slideserve.com
PPT Titrimetric Analysis PowerPoint Presentation, free download ID Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in chemistry, titrimetric methods are broadly classified into three types: titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s). Titrimetric Analysis Lab.
From bramblechemistry.weebly.com
4C6 Titration Titrimetric Analysis Lab Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in this document, we will examine some of the specifics of titrimetric analysis: titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. to be suitable for a titration, the analyte and titrant must undergo a rapid redox. Titrimetric Analysis Lab.
From collegedunia.com
Volumetric Analysis Titration, Types, Principle & Procedure Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in chemistry, titrimetric methods are broadly classified into three types: in this document, we will examine some of the specifics of titrimetric analysis: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative. Titrimetric Analysis Lab.
From www.slideserve.com
PPT Principles of Volumetric (Titrimetric) Analysis PowerPoint Titrimetric Analysis Lab in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has. Titrimetric Analysis Lab.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. to be suitable for a titration, the analyte and titrant must. Titrimetric Analysis Lab.
From www.microlit.com
An Advanced Guide to Titration Microlit Titrimetric Analysis Lab Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in chemistry, titrimetric methods are broadly classified into three types: in this document, we will examine some of the specifics of titrimetric analysis: titrimetric analysis involves determination of. Titrimetric Analysis Lab.
From thechemistrynotes.com
Titrimetric Analysis Terms, Types, Principle, Advantages Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in this document, we will examine some of the specifics of titrimetric analysis: in chemistry, titrimetric methods are broadly classified into three types: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative. Titrimetric Analysis Lab.
From www.dreamstime.com
Chemical Laboratory. Hand with Flask during Titrimetric Analysis or Titrimetric Analysis Lab in chemistry, titrimetric methods are broadly classified into three types: in this document, we will examine some of the specifics of titrimetric analysis: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction. Titrimetric Analysis Lab.
From www.studocu.com
Chemistry Lab 8 Chem150L Titrimetric Analysis ** Scroll right to find Titrimetric Analysis Lab in this document, we will examine some of the specifics of titrimetric analysis: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in chemistry, titrimetric methods are broadly classified into three types: titrimetric analysis involves determination of. Titrimetric Analysis Lab.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titrimetric Analysis Lab in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in this document, we will examine some of the specifics of titrimetric analysis: titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. titration (also known as. Titrimetric Analysis Lab.
From www.coursehero.com
. POSTLABORATORY REPORT EXPERIMENT NO. 5 Titrimetric Analysis Titrimetric Analysis Lab titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. . Titrimetric Analysis Lab.
From www.pmf.ni.ac.rs
Titrimetric Analysis Chemia Naissensis Titrimetric Analysis Lab in this document, we will examine some of the specifics of titrimetric analysis: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in chemistry, titrimetric methods are broadly classified into three types: titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is. Titrimetric Analysis Lab.
From www.youtube.com
Lab Experiment 5 Volumetric Analysis by RedOx Titration. YouTube Titrimetric Analysis Lab titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in this document, we will examine some of the specifics of titrimetric analysis: in chemistry, titrimetric methods are broadly classified into three types: titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is. Titrimetric Analysis Lab.
From gioldvmyt.blob.core.windows.net
Titration Chemical Method at Karl Twiggs blog Titrimetric Analysis Lab Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in chemistry, titrimetric methods are broadly classified into three types: titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a. Titrimetric Analysis Lab.
From www.studocu.com
Titrimetric Analysis TITRIMETRIC ANALYSIS Standard Solution Titration Titrimetric Analysis Lab Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in chemistry, titrimetric methods are broadly classified into three types: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in this document, we will examine some of the specifics of. Titrimetric Analysis Lab.
From gioajmlyz.blob.core.windows.net
Titration Chemistry Research at Nicole Apple blog Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a. Titrimetric Analysis Lab.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titrimetric Analysis Lab titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in chemistry, titrimetric methods are broadly classified into three types: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s). Titrimetric Analysis Lab.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titrimetric Analysis Lab titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in chemistry, titrimetric methods are broadly classified into three types: . Titrimetric Analysis Lab.
From www.shutterstock.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Titrimetric Analysis Lab to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which is required to. in chemistry, titrimetric methods are broadly classified into three types: Ag+(aq) +scn−(aq) ⇌ ag(scn)(s). Titrimetric Analysis Lab.
From hxetahfoe.blob.core.windows.net
Analysis Titration at Pauline Estill blog Titrimetric Analysis Lab Ag+(aq) +scn−(aq) ⇌ ag(scn)(s) a g + (a q) + s. in this document, we will examine some of the specifics of titrimetric analysis: to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. in chemistry, titrimetric methods are broadly classified into. Titrimetric Analysis Lab.
From www.studocu.com
Lab Expt 7 Titrimetric Analysis Analytical Chemistry Laboratory Term Titrimetric Analysis Lab titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. to be suitable for a titration, the analyte and titrant must undergo a rapid redox reaction that has a very large equilibrium constant so that. titrimetric analysis involves determination of the volume of a solution of accurately known concentration, which. Titrimetric Analysis Lab.