Chlorine Dioxide Synthesis . The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. preparation of pure chlorine dioxide.
from www.alamy.com
The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Reactions of chlorine or sodium hypochlorite with sodium. experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. preparation of pure chlorine dioxide. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid.
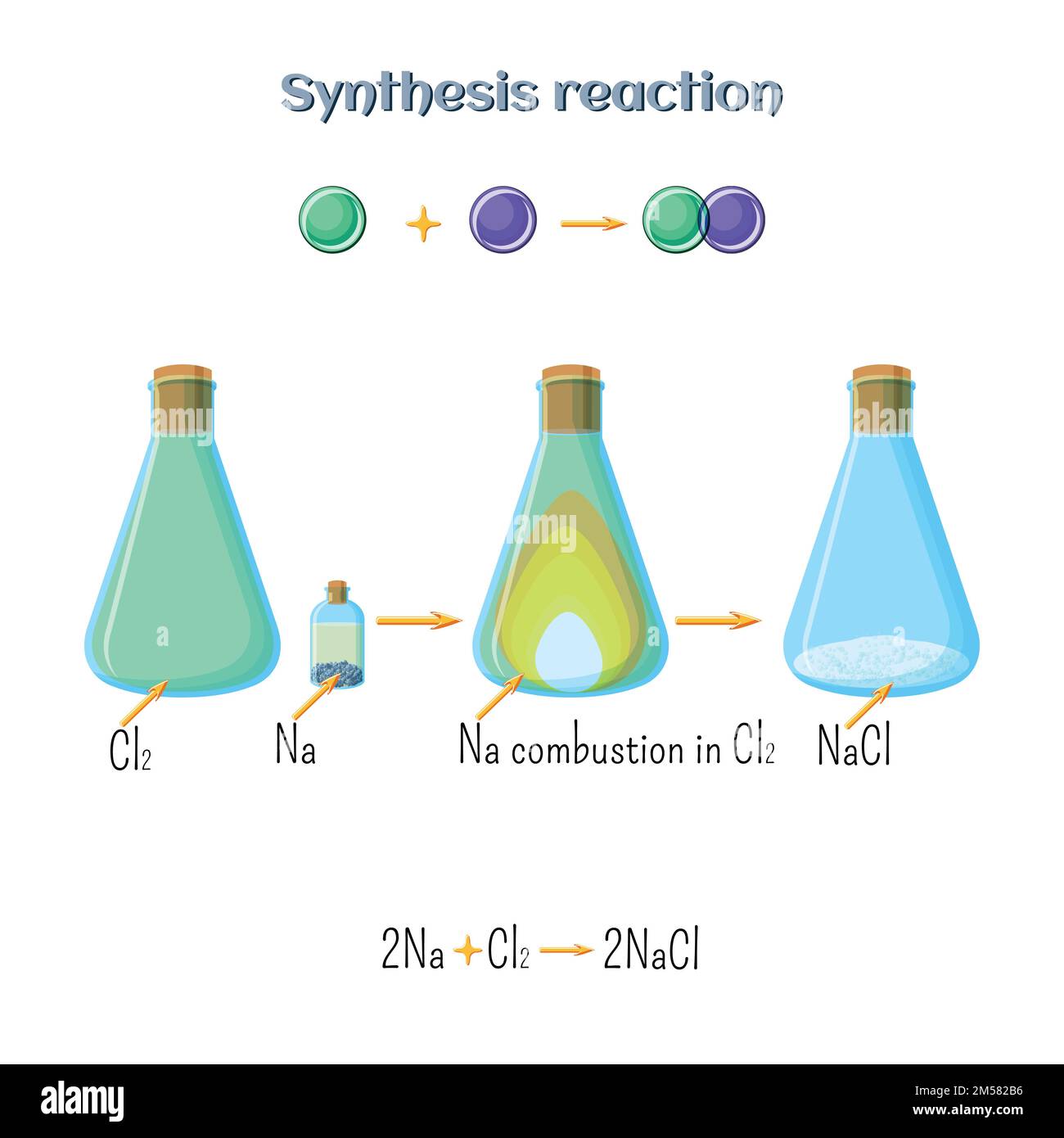
Synthesis reaction sodium chloride formation of sodium metal and
Chlorine Dioxide Synthesis Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. preparation of pure chlorine dioxide. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Reactions of chlorine or sodium hypochlorite with sodium.
From feedwater.co.uk
Chlorine Dioxide Water Treatment Feedwater site Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. . Chlorine Dioxide Synthesis.
From cartoondealer.com
Chlorine Dioxide, ClO2, Ballandstick Model, Molecular And Chemical Chlorine Dioxide Synthesis The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. chlorine dioxide is always prepared. Chlorine Dioxide Synthesis.
From www.semanticscholar.org
Figure 1 from Reactions of Chlorine Dioxide with Organic Compounds Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. experiments have shown that high. Chlorine Dioxide Synthesis.
From www.researchgate.net
Scheme 13 Reaction of chlorine dioxide with ketosulfides 4249 Chlorine Dioxide Synthesis preparation of pure chlorine dioxide. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components.. Chlorine Dioxide Synthesis.
From hoachatdackhang.com
[Bán] Hóa Chất Chlorine Dioxide Giá Tốt, Uy Tín Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Reactions of chlorine or sodium hypochlorite with sodium. preparation of pure chlorine dioxide.. Chlorine Dioxide Synthesis.
From idiclo2.com
About Chlorine Dioxide International Dioxide Chlorine Dioxide Synthesis Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. . Chlorine Dioxide Synthesis.
From mastermineralsolutions.com
Disinfectant Chlorine Dioxide MMS Master Mineral Solutions Chlorine Dioxide Synthesis experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine or sodium hypochlorite with sodium. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components.. Chlorine Dioxide Synthesis.
From www.pall.com
Chlorine Dioxide Pulp Bleaching & Paper Chemical Preparation Pall Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. experiments have shown that high temperature, high ph and high initial concentration accelerate the. preparation of pure chlorine dioxide. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine. Chlorine Dioxide Synthesis.
From www.alamy.com
Synthesis reaction sodium chloride formation of sodium metal and Chlorine Dioxide Synthesis Reactions of chlorine or sodium hypochlorite with sodium. experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine. Chlorine Dioxide Synthesis.
From cartoondealer.com
Chlorine Dioxide, ClO2, Ballandstick Model, Molecular And Chemical Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. preparation of pure chlorine dioxide. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine. Chlorine Dioxide Synthesis.
From www.watertechusa.com
Supplemental Disinfection Watertech of America, Inc. serving WI, IL Chlorine Dioxide Synthesis The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid.. Chlorine Dioxide Synthesis.
From dokumen.tips
(PDF) Oxidations of amines. X. Detailed in the reaction of Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine or sodium hypochlorite with sodium. preparation of pure chlorine dioxide. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Chlorine dioxide (clo2) is produced from the oxidation of sodium. Chlorine Dioxide Synthesis.
From one-a.zhihuiya.com
Chlorine dioxide solid sustainedrelease agent and preparation method Chlorine Dioxide Synthesis experiments have shown that high temperature, high ph and high initial concentration accelerate the. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. preparation of pure chlorine dioxide. Chlorine dioxide (clo2) is produced from the oxidation of sodium. Chlorine Dioxide Synthesis.
From www.trekkinn.com
Lifesystems Chlorine Dioxide Liquid 2 X 30ml Black, Trekkinn Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. preparation of pure chlorine dioxide. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite. Chlorine Dioxide Synthesis.
From www.cdslab.es
How is Chlorine Dioxide prepared? Clorito de sodio, dioxido de cloro Chlorine Dioxide Synthesis experiments have shown that high temperature, high ph and high initial concentration accelerate the. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. preparation of pure chlorine dioxide.. Chlorine Dioxide Synthesis.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Chlorine Dioxide Synthesis Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. preparation of pure chlorine dioxide. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Reactions of chlorine or sodium hypochlorite with sodium.. Chlorine Dioxide Synthesis.
From www.researchgate.net
Schematic of R8 chlorine dioxide generator showing various inputs and Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Reactions of chlorine or sodium hypochlorite with sodium. The invention provides a process for producing. Chlorine Dioxide Synthesis.
From eureka.patsnap.com
Chlorine dioxide generator for the efficient generation of chlorine Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. experiments have shown that high temperature, high ph and high initial concentration accelerate the. Chlorine dioxide (clo2) is produced from the oxidation of. Chlorine Dioxide Synthesis.
From www.alamy.com
Diagram of the laboratory preparation of carbon dioxide from Stock Chlorine Dioxide Synthesis preparation of pure chlorine dioxide. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide is always prepared as a solution on site via oxidation of sodium. Chlorine Dioxide Synthesis.
From chemicalnote.com
Sulphur dioxide (SO2) Preparation, Properties and Uses. Chlorine Dioxide Synthesis experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. The. Chlorine Dioxide Synthesis.
From www.alamy.com
Chlorine dioxide (ClO2) molecule. Used in pulp bleaching and for Chlorine Dioxide Synthesis experiments have shown that high temperature, high ph and high initial concentration accelerate the. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. preparation of pure chlorine dioxide. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. chlorine dioxide is always prepared as a solution. Chlorine Dioxide Synthesis.
From manepa.com
PureChlor Compact Chlorine Dioxide System Manepa Medical Chlorine Dioxide Synthesis Reactions of chlorine or sodium hypochlorite with sodium. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. experiments have shown that high temperature, high ph and high initial concentration accelerate the. preparation of pure chlorine dioxide. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite. Chlorine Dioxide Synthesis.
From www.numerade.com
SOLVED Chlorine is prepared in the laboratory bytreating manganese Chlorine Dioxide Synthesis Reactions of chlorine or sodium hypochlorite with sodium. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. preparation of pure chlorine dioxide. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. . Chlorine Dioxide Synthesis.
From www.vecteezy.com
Preparation of chlorine in laboratory. vector image illustration Chlorine Dioxide Synthesis Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide can be efficiently produced from hydrogen. Chlorine Dioxide Synthesis.
From www.alamy.com
Chlorine dioxide (ClO2) molecule. Used in pulp bleaching and for Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. preparation of pure chlorine dioxide. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. The invention provides. Chlorine Dioxide Synthesis.
From www.scientific.net
of the Reaction for Generation of Chlorine Dioxide from Sodium Chlorine Dioxide Synthesis Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine or sodium hypochlorite with sodium.. Chlorine Dioxide Synthesis.
From www.linkedin.com
Disinfection Mechanism of Chlorine Dioxide (CIO2) Chlorine Dioxide Synthesis preparation of pure chlorine dioxide. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Reactions of chlorine or sodium hypochlorite with sodium. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid.. Chlorine Dioxide Synthesis.
From www.youtube.com
Preparation of Chlorine gas in lab. diagram making video YouTube Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. preparation of pure chlorine dioxide. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium.. Chlorine Dioxide Synthesis.
From www.vitasoul.co.za
CDS Chlorine Dioxide Solution VitaSoul Chlorine Dioxide Synthesis Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. preparation of pure chlorine dioxide. experiments have shown that high temperature, high ph and high initial concentration accelerate the. The invention provides. Chlorine Dioxide Synthesis.
From chlorinedioxidesolution.ca
Chlorine Dioxide Formula chlorinedioxide Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. preparation of pure chlorine dioxide. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide. Chlorine Dioxide Synthesis.
From brainly.in
1)Draw a labelled diagram to show the preparation of hydrogen chloride Chlorine Dioxide Synthesis preparation of pure chlorine dioxide. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. Reactions of chlorine or sodium hypochlorite with sodium. experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media.. Chlorine Dioxide Synthesis.
From eureka.patsnap.com
Chlorine dioxide fumigation disinfectant for automobile air Chlorine Dioxide Synthesis chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. preparation of pure chlorine dioxide. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. experiments have shown that high temperature, high ph and high initial concentration accelerate the. chlorine dioxide is always prepared as a solution. Chlorine Dioxide Synthesis.
From www.dioxide.com
Chlorine Dioxide Generation and Dosing Systems chemical and echem Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Reactions of chlorine or sodium hypochlorite with sodium. preparation of pure chlorine dioxide. The invention provides a process for producing chlorine dioxide by. Chlorine Dioxide Synthesis.
From www.dreamstime.com
3D Image of Chlorine Dioxide Skeletal Formula Stock Illustration Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Chlorine dioxide (clo2) is produced from the oxidation of sodium chlorite (naclo2) by hypochlorous acid. Reactions of chlorine or sodium hypochlorite with sodium. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components.. Chlorine Dioxide Synthesis.
From eureka.patsnap.com
Chlorine dioxide composition and preparing method thereof Eureka Chlorine Dioxide Synthesis chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid. Reactions of chlorine or sodium hypochlorite with sodium. The invention provides a process for producing chlorine dioxide by the reaction of two precursor components. preparation of pure chlorine dioxide. Chlorine dioxide (clo2) is produced from the oxidation of. Chlorine Dioxide Synthesis.