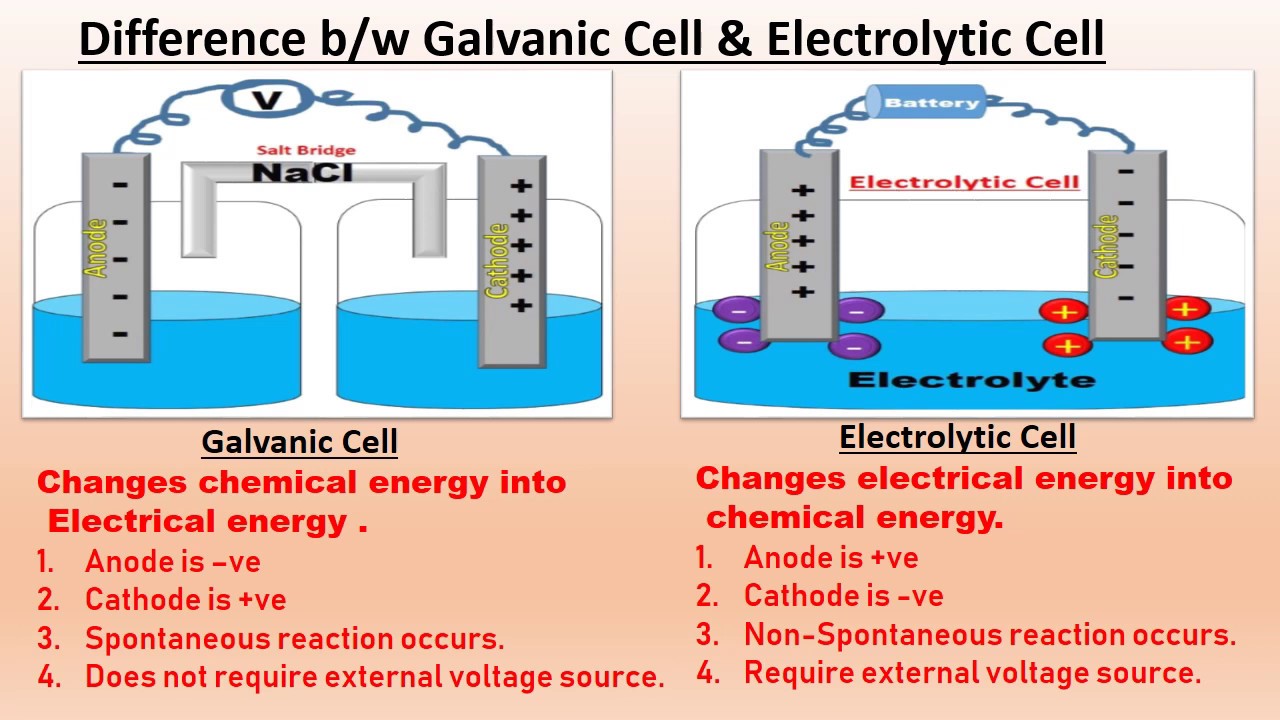
The Galvanic Cell Reaction . a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Abbreviated symbolism is commonly used to.
from www.aiophotoz.com
Abbreviated symbolism is commonly used to. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions.
What Is The Similarities And Differences Between Galvanic Cell And
The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to.
From www.pinterest.com.au
What is Galvanic Cell? Galvanic cell, Electrochemistry, Science biology The Galvanic Cell Reaction Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical. The Galvanic Cell Reaction.
From lessonschoolallodial.z13.web.core.windows.net
Electrochemical Cells In Chemistry The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell based on the spontaneous reaction between copper and silver (i). The Galvanic Cell Reaction.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From schematicdiagramyakuza.z13.web.core.windows.net
Alkaline Cell Diagram The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the galvanic cell, or called voltaic cell, is an electrochemical. The Galvanic Cell Reaction.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a. The Galvanic Cell Reaction.
From www.aiophotoz.com
What Is The Similarities And Differences Between Galvanic Cell And The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices. The Galvanic Cell Reaction.
From www.youtube.com
Find the cell potential of a galvanic cell based on the following The Galvanic Cell Reaction the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices. The Galvanic Cell Reaction.
From diagramdatablashiest.z5.web.core.windows.net
Electrochemical Cell Diagram The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Abbreviated symbolism is commonly used to. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the energy released during a. The Galvanic Cell Reaction.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary The Galvanic Cell Reaction Abbreviated symbolism is commonly used to. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From mavink.com
Galvanic Cell Labeled The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. a galvanic cell based on the spontaneous reaction. The Galvanic Cell Reaction.
From learningschoolletopisaln.z22.web.core.windows.net
Types Of Electrochemical Cells Pdf The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a. The Galvanic Cell Reaction.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From khmerrapsp5schematic.z14.web.core.windows.net
What Happens At The Cathode In Electrolysis The Galvanic Cell Reaction the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. devices of this sort are generally referred to as electrochemical cells, and those in. The Galvanic Cell Reaction.
From wirepartserjeantcy.z21.web.core.windows.net
How To Draw An Electrochemical Cell The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in. The Galvanic Cell Reaction.
From www.youtube.com
Galvanic cell or Voltaic cell Definition ,Construction and Working The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. the galvanic cell, or called voltaic cell, is. The Galvanic Cell Reaction.
From lessonschoolallodial.z13.web.core.windows.net
Electrochemical Cells In Chemistry The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. a galvanic cell based on the spontaneous reaction. The Galvanic Cell Reaction.
From schematicdiagramyakuza.z13.web.core.windows.net
Chemical Cell Diagram The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. Abbreviated symbolism is commonly used to. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From circuitwiringtray.z13.web.core.windows.net
Chemical Cell Diagram The Galvanic Cell Reaction the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From kell2414h2schematic.z21.web.core.windows.net
Electrochemistry Cell Diagram The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Abbreviated symbolism is commonly used to. a galvanic (voltaic) cell uses the energy. The Galvanic Cell Reaction.
From www.vrogue.co
What Is A Galvanic Cell Definition From Corrosionpedi vrogue.co The Galvanic Cell Reaction the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to. The Galvanic Cell Reaction.
From wps.prenhall.com
Media Portfolio The Galvanic Cell Reaction Abbreviated symbolism is commonly used to. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices. The Galvanic Cell Reaction.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE The Galvanic Cell Reaction Abbreviated symbolism is commonly used to. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells,. The Galvanic Cell Reaction.
From ceaujltn.blob.core.windows.net
Zinc Copper Galvanic Cell Half Reactions at David Deans blog The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. the galvanic cell, or called voltaic cell,. The Galvanic Cell Reaction.
From saylordotorg.github.io
Describing Electrochemical Cells The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. a galvanic cell based on the spontaneous reaction. The Galvanic Cell Reaction.
From 2012books.lardbucket.org
Oxidation and Reduction The Galvanic Cell Reaction a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From chem.libretexts.org
Chapter 19.5 Commercial Galvanic Cells Chemistry LibreTexts The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. the galvanic cell, or called voltaic cell,. The Galvanic Cell Reaction.
From circuitsbrescia8g.z22.web.core.windows.net
Corrosion Circuit Diagram The Galvanic Cell Reaction the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices. The Galvanic Cell Reaction.
From circuitbogodufu.z4.web.core.windows.net
Simple Electric Cell Diagram The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. Abbreviated symbolism is commonly used to. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the. The Galvanic Cell Reaction.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps The Galvanic Cell Reaction Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. the galvanic cell, or called voltaic cell,. The Galvanic Cell Reaction.
From schematicljubkujei6.z22.web.core.windows.net
Cell Diagram Notation The Galvanic Cell Reaction the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Abbreviated symbolism is commonly used to. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From courses.lumenlearning.com
Galvanic Cells Chemistry for Majors The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Abbreviated symbolism is commonly used to. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From wirepartserjeantcy.z21.web.core.windows.net
Simple Cell Diagram Chemistry The Galvanic Cell Reaction Abbreviated symbolism is commonly used to. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous. The Galvanic Cell Reaction.
From chemistry.stackexchange.com
electrochemistry Galvanic cells/redox confused regarding diagram The Galvanic Cell Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. a galvanic cell based on the spontaneous reaction between copper and silver (i). The Galvanic Cell Reaction.
From wiringfixmashedpatatasz3.z14.web.core.windows.net
Cathode Charge In Electrolysis The Galvanic Cell Reaction a galvanic cell based on the spontaneous reaction between copper and silver (i) ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell.. The Galvanic Cell Reaction.