Titration Indicator Potassium Dichromate . dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. The potassium dichromate oxidizes iron (ii) to iron. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. There are three indicators that may be used for the. unlike permanganate, dichromate titrations require an indicator. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by.
from slidetodoc.com
suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. There are three indicators that may be used for the. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. The potassium dichromate oxidizes iron (ii) to iron. unlike permanganate, dichromate titrations require an indicator.
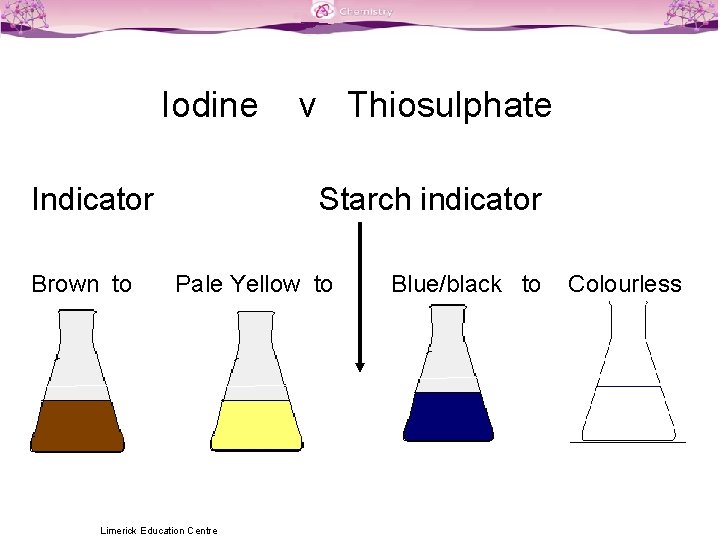
Titration Colour Changes SLSS Science Limerick Education Centre
Titration Indicator Potassium Dichromate the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. There are three indicators that may be used for the. In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by. unlike permanganate, dichromate titrations require an indicator. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. The potassium dichromate oxidizes iron (ii) to iron.
From www.youtube.com
Potassium Dichromate Titration YouTube Titration Indicator Potassium Dichromate The potassium dichromate oxidizes iron (ii) to iron. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. unlike permanganate, dichromate titrations require an indicator. There are three. Titration Indicator Potassium Dichromate.
From www.studocu.com
Exp5 Practical CHEMISTRY LABORATORY [CHEM 102] EXPERIMENT NO. 05 Titration Indicator Potassium Dichromate There are three indicators that may be used for the. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. unlike permanganate, dichromate titrations require an indicator. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. dichrometry is a type of redox titration that uses potassium. Titration Indicator Potassium Dichromate.
From www.toppr.com
What is correct increasing order of bond lengths of bond indicated as I Titration Indicator Potassium Dichromate The potassium dichromate oxidizes iron (ii) to iron. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. There are three indicators that may be used for the. there are three indicators that may be used for the. Titration Indicator Potassium Dichromate.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Indicator Potassium Dichromate There are three indicators that may be used for the. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. The potassium dichromate oxidizes iron (ii) to iron. unlike permanganate, dichromate titrations require an indicator. the potassium. Titration Indicator Potassium Dichromate.
From fphoto.photoshelter.com
potassium dichromate sulfuric acid color change Fundamental Titration Indicator Potassium Dichromate dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. unlike permanganate, dichromate titrations require an indicator. In this experiment you will use a standard solution of potassium dichromate (k. Titration Indicator Potassium Dichromate.
From www.youtube.com
The colour change of acidified Potassium Dichromate The Real Chemist Titration Indicator Potassium Dichromate dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. The potassium dichromate oxidizes iron (ii) to iron. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. unlike permanganate, dichromate titrations require an. Titration Indicator Potassium Dichromate.
From www.numerade.com
SOLVED Potassium dichromate is an oxidizing agent that is used for the Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. The potassium dichromate oxidizes iron (ii) to iron. dichrometry is a type. Titration Indicator Potassium Dichromate.
From www.youtube.com
Silver Nitrate (AgNO3) Reaction With Potassium dichromate (K2Cr2O7 Titration Indicator Potassium Dichromate the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. The potassium dichromate oxidizes iron (ii) to iron. In this experiment you will. Titration Indicator Potassium Dichromate.
From cegtwwll.blob.core.windows.net
Indicator Work In A Titration at Amy Tanner blog Titration Indicator Potassium Dichromate there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. The potassium dichromate oxidizes iron (ii) to. Titration Indicator Potassium Dichromate.
From www.lazada.com.ph
Potassium dichromate standard solution titration analysis COD detection Titration Indicator Potassium Dichromate suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. The potassium dichromate oxidizes iron (ii) to iron. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. There are three indicators. Titration Indicator Potassium Dichromate.
From www.slideserve.com
PPT Variable oxidation states PowerPoint Presentation ID3450341 Titration Indicator Potassium Dichromate dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability. Titration Indicator Potassium Dichromate.
From www.fishersci.ca
Ricca Chemical Company Potassium Chromate, 5 (w/v), Chloride Free Titration Indicator Potassium Dichromate In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. . Titration Indicator Potassium Dichromate.
From hoakhoa.com.vn
Potassium dichromate volumetric standard 1024030080 Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. The potassium dichromate oxidizes iron (ii) to iron. unlike permanganate, dichromate titrations require an indicator. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. dichrometry is a type of redox titration that. Titration Indicator Potassium Dichromate.
From slideplayer.com
Redox titration Lecture 9 Associate prof . L.V. Vronska ppt download Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. unlike permanganate,. Titration Indicator Potassium Dichromate.
From www.numerade.com
SOLVED Consider a titration of potassium dichromate solution with Titration Indicator Potassium Dichromate dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. the potassium dichromate solution is slowly added from a burette into. Titration Indicator Potassium Dichromate.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Titration Indicator Potassium Dichromate there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. unlike permanganate, dichromate titrations require an indicator. There are three indicators that may be used for the. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. dichrometry is a type of redox titration. Titration Indicator Potassium Dichromate.
From www.numerade.com
SOLVED The iron content of an ore sample can be determined by redox Titration Indicator Potassium Dichromate there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. There are three indicators that may be used for the. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. the potassium dichromate solution is slowly added from. Titration Indicator Potassium Dichromate.
From ceyuitwn.blob.core.windows.net
Indicator Titration Purpose at Richard Neumann blog Titration Indicator Potassium Dichromate unlike permanganate, dichromate titrations require an indicator. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. the potassium dichromate solution is slowly added from a burette. Titration Indicator Potassium Dichromate.
From www.numerade.com
SOLVED(A) Starch is the indicator used in the iodometric titrations Titration Indicator Potassium Dichromate unlike permanganate, dichromate titrations require an indicator. There are three indicators that may be used for the. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. The potassium dichromate oxidizes iron (ii) to iron. the potassium dichromate solution. Titration Indicator Potassium Dichromate.
From cegtwwll.blob.core.windows.net
Indicator Work In A Titration at Amy Tanner blog Titration Indicator Potassium Dichromate The potassium dichromate oxidizes iron (ii) to iron. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. dichrometry is a type. Titration Indicator Potassium Dichromate.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID2145720 Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. The potassium dichromate oxidizes iron (ii) to iron. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. unlike permanganate, dichromate titrations. Titration Indicator Potassium Dichromate.
From www.youtube.com
Chem Expt 8 DichromateChromate Equilibrium (pH dependent) YouTube Titration Indicator Potassium Dichromate In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. The potassium dichromate oxidizes iron (ii) to iron. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are. Titration Indicator Potassium Dichromate.
From www.researchgate.net
Can Potassium dichromate be used as self indicator in redox titrations Titration Indicator Potassium Dichromate suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. There are three indicators that may be used for the. unlike permanganate, dichromate titrations require an indicator. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. the potassium dichromate solution is slowly added from a burette into the. Titration Indicator Potassium Dichromate.
From www.academia.edu
(DOC) Experiment no. 1 Iodometric titration of potassium dichromate Titration Indicator Potassium Dichromate There are three indicators that may be used for the. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. potassium dichromate is a relatively strong oxidizing agent whose principal advantages. Titration Indicator Potassium Dichromate.
From www.coursehero.com
[Solved] balanced reaction between ethanol and potassium dichromate Titration Indicator Potassium Dichromate there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. In this. Titration Indicator Potassium Dichromate.
From www.toppr.com
Consider a titration of potassium dichromate solution with scified Mone Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. There are three indicators that may be used for the. unlike permanganate,. Titration Indicator Potassium Dichromate.
From www.youtube.com
Titration of Mohr Salt Solution with Standard Potassium Dichromate Titration Indicator Potassium Dichromate In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. The potassium dichromate oxidizes iron (ii) to iron. the potassium dichromate solution is slowly added from a burette into. Titration Indicator Potassium Dichromate.
From slideplayer.com
Titration Colour Changes ppt download Titration Indicator Potassium Dichromate dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. unlike permanganate, dichromate titrations require an indicator. There are three indicators that may be used for the. In this experiment. Titration Indicator Potassium Dichromate.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate sodium dichromate Titration Indicator Potassium Dichromate The potassium dichromate oxidizes iron (ii) to iron. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. There are three indicators that may be used for the. suitable indicators for. Titration Indicator Potassium Dichromate.
From www.reddit.com
Determination of Fe2+ in a solution through gravimetric titration Titration Indicator Potassium Dichromate There are three indicators that may be used for the. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. The potassium dichromate oxidizes iron (ii) to iron. unlike permanganate, dichromate titrations require an indicator. there are three indicators that may be used for the titration of fe 2+ with k 2. Titration Indicator Potassium Dichromate.
From www.lazada.com.ph
0.1mol/L Potassium Dichromate Standard Solution Titration Analysis Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as the oxidizing agent. The potassium dichromate oxidizes iron (ii) to iron. unlike permanganate, dichromate titrations require an indicator. There are three indicators that may be used. Titration Indicator Potassium Dichromate.
From www.youtube.com
Titration of potassium dichromate with ferrous ammonium sulphate, B.Sc Titration Indicator Potassium Dichromate The potassium dichromate oxidizes iron (ii) to iron. In this experiment you will use a standard solution of potassium dichromate (k 2 cr 2 o 7) to determine the percent by. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. suitable indicators for dichromate titrations are. Titration Indicator Potassium Dichromate.
From www.toppr.com
Consider a titration of potassium dichromate solution with acidified Titration Indicator Potassium Dichromate suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. unlike permanganate, dichromate titrations require an indicator. There are three indicators that may be used for the. dichrometry is a type of redox titration that uses potassium dichromate (k2cr2o7) as. Titration Indicator Potassium Dichromate.
From www.numerade.com
SOLVEDPotassium dichromate is an oxidizing agent that is used for the Titration Indicator Potassium Dichromate potassium dichromate is a relatively strong oxidizing agent whose principal advantages are its availability as a primary standard. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. suitable indicators for dichromate titrations are diphenylamine (specifically sodium diphenylamine sulphonate)(0.2%aq. There are three indicators that may be used for the. In this experiment. Titration Indicator Potassium Dichromate.
From questions-in.kunduz.com
002 00 62. 3) 4) Consider a titration Physical Chemistry Titration Indicator Potassium Dichromate There are three indicators that may be used for the. the potassium dichromate solution is slowly added from a burette into the flask, continuously swirling. unlike permanganate, dichromate titrations require an indicator. there are three indicators that may be used for the titration of fe 2+ with k 2 cr 2 o 7. dichrometry is a. Titration Indicator Potassium Dichromate.