Which Of These Is Most Soluble In Water . use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. All the options given to us in the above question are isomeric alcohol which. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. A salt is soluble if it dissolves in water to give a solution. which of the following is most soluble in water? complete step by step solution:
from www.studyxapp.com
rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. which of the following is most soluble in water? complete step by step solution: hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. All the options given to us in the above question are isomeric alcohol which. A salt is soluble if it dissolves in water to give a solution.
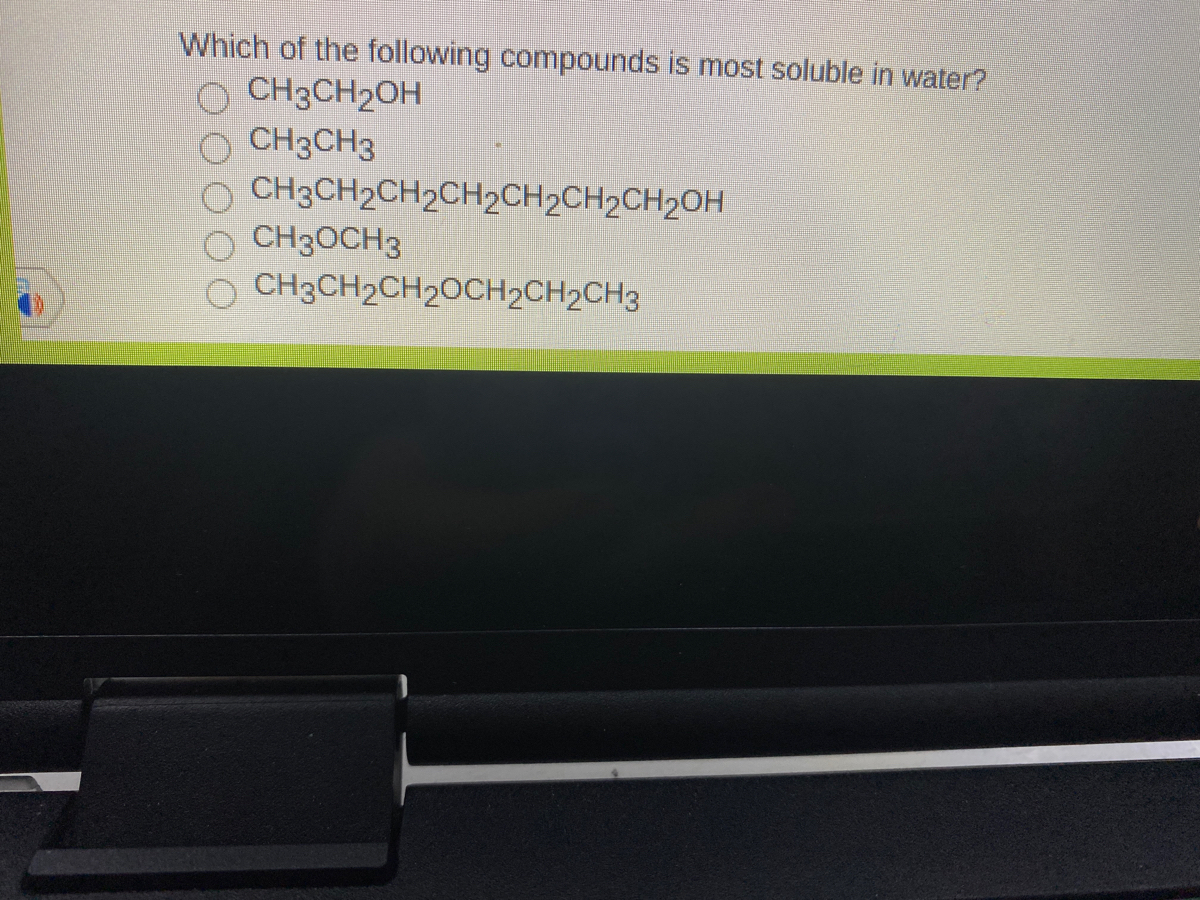
which of the following compounds is most soluble in watero ch3ch2oho
Which Of These Is Most Soluble In Water which of the following is most soluble in water? All the options given to us in the above question are isomeric alcohol which. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. which of the following is most soluble in water? A salt is soluble if it dissolves in water to give a solution. complete step by step solution: use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble.
From ulisesfersburns.blogspot.com
Identify Which of the Following Is Soluble in Water. Which Of These Is Most Soluble In Water hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. All the options given to us in the above question are isomeric alcohol which. A. Which Of These Is Most Soluble In Water.
From www.coursehero.com
[Solved] Which of the following compounds is least soluble in water? O Which Of These Is Most Soluble In Water All the options given to us in the above question are isomeric alcohol which. complete step by step solution: these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. A salt is soluble if it dissolves in water to give a solution. rubidium formate, thallium formate, and silver perchlorate are 3. Which Of These Is Most Soluble In Water.
From www.bartleby.com
Answered 1. Which molecule is most soluble in… bartleby Which Of These Is Most Soluble In Water hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. A salt is soluble if it dissolves in water to give a solution. All the options given to. Which Of These Is Most Soluble In Water.
From www.numerade.com
SOLVED Which compound is the MOST soluble in water? OH "OH Which Of These Is Most Soluble In Water All the options given to us in the above question are isomeric alcohol which. complete step by step solution: hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. A salt is soluble if it dissolves in water to give a solution. which of the following. Which Of These Is Most Soluble In Water.
From www.chegg.com
Chemistry Archive March 19, 2016 Which Of These Is Most Soluble In Water hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. complete step by step solution: A salt is soluble if it dissolves in water to give a solution. use. Which Of These Is Most Soluble In Water.
From www.numerade.com
SOLVED Which of these molecules would be most soluble in water? Which Of These Is Most Soluble In Water A salt is soluble if it dissolves in water to give a solution. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. All the options given to us in the. Which Of These Is Most Soluble In Water.
From solvedlib.com
Which of the following compounds is most soluble in w… SolvedLib Which Of These Is Most Soluble In Water A salt is soluble if it dissolves in water to give a solution. which of the following is most soluble in water? All the options given to us in the above question are isomeric alcohol which. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. rubidium formate, thallium formate, and. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved of the following compounds will be more soluble in Which Of These Is Most Soluble In Water use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. complete step by step solution: which of the following is most soluble in water? rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. A. Which Of These Is Most Soluble In Water.
From www.numerade.com
SOLVED which is most soluble in water? which is least soluble? explain Which Of These Is Most Soluble In Water which of the following is most soluble in water? hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. complete step by step solution: use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. these. Which Of These Is Most Soluble In Water.
From viaterra.mx
Introducir 33+ images la pintura es soluble o insoluble Viaterra.mx Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. which of the following is most soluble in water? these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. use the solubility rules to predict if a compound. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Rank these compounds from most soluble in water to Which Of These Is Most Soluble In Water complete step by step solution: A salt is soluble if it dissolves in water to give a solution. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. All the options given to us in the above question are isomeric alcohol which. use the solubility. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Which compound is the most soluble in water? Which Of These Is Most Soluble In Water A salt is soluble if it dissolves in water to give a solution. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. hydrophilic substances tend to be very soluble in water. Which Of These Is Most Soluble In Water.
From rayb78.github.io
Solubility In Water Chart Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. A salt is soluble if it dissolves in water to give a solution. All the. Which Of These Is Most Soluble In Water.
From askfilo.com
Which of these ionic compounds is most soluble in water Filo Which Of These Is Most Soluble In Water these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. . Which Of These Is Most Soluble In Water.
From www.numerade.com
SOLVEDIdentify the compound in each group that is most soluble in Which Of These Is Most Soluble In Water use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. A salt is soluble if it dissolves in water to give a solution. All the options given to us in the above question are isomeric alcohol which. hydrophilic substances tend to be very soluble in water and other strongly. Which Of These Is Most Soluble In Water.
From www.prepladder.com
Water Soluble Vitamins NEET PG Biochemistry Which Of These Is Most Soluble In Water which of the following is most soluble in water? hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. All the options given to us in the above question are. Which Of These Is Most Soluble In Water.
From www.youtube.com
which molecule is most soluble in water? YouTube Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. All the options given to us in the above question are isomeric alcohol which. A salt is soluble if it dissolves in water to give a solution. these rules are based on the following definitions of. Which Of These Is Most Soluble In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Which Of These Is Most Soluble In Water complete step by step solution: A salt is soluble if it dissolves in water to give a solution. which of the following is most soluble in water? hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. All the options given to us in the above. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Which of these compounds is the most soluble in Which Of These Is Most Soluble In Water All the options given to us in the above question are isomeric alcohol which. which of the following is most soluble in water? rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. hydrophilic substances tend to be very soluble in water and other strongly. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Which of the following is most soluble in water? Which Of These Is Most Soluble In Water these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. A salt is soluble if it dissolves in water to give a solution. which of the following is most soluble in water?. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Which of the following molecules is most soluble in Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. complete step by step solution: All the options given to us in the above question are isomeric alcohol which. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when. Which Of These Is Most Soluble In Water.
From exogqaefj.blob.core.windows.net
Copper Chloride Water Solubility at Sandra Barrera blog Which Of These Is Most Soluble In Water hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. which of the following is most soluble in water? A salt is soluble if it dissolves in water to give a solution. complete step by step solution: rubidium formate, thallium formate, and silver perchlorate are. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Which compound will be the most soluble in water? Which Of These Is Most Soluble In Water these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially. Which Of These Is Most Soluble In Water.
From www.numerade.com
SOLVED My answer is wrong L Question 4 of 5 > Attempt 2 4PKET Which Of These Is Most Soluble In Water hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. complete step by step solution: which of the following is most soluble in water? All the options given to us in the above question are isomeric alcohol which. A salt is soluble if it dissolves in. Which Of These Is Most Soluble In Water.
From oneclass.com
OneClass Rank the following organic compounds from most soluble in Which Of These Is Most Soluble In Water use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. which of the following is most soluble in water? hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. A salt is soluble if it dissolves in. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Rank These Compounds From Most Soluble In Water To... Which Of These Is Most Soluble In Water All the options given to us in the above question are isomeric alcohol which. which of the following is most soluble in water? A salt is soluble if it dissolves in water to give a solution. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving.. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Rank these compounds from most soluble in water to Which Of These Is Most Soluble In Water hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. All the options given to us in the above question are isomeric alcohol which. A salt is soluble if it dissolves. Which Of These Is Most Soluble In Water.
From jovany-has-english.blogspot.com
Which of the Following Is Least Soluble in Water JovanyhasEnglish Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. which of the following is most soluble in water? these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. A salt is soluble if it dissolves in water to. Which Of These Is Most Soluble In Water.
From www.studyxapp.com
which of the following compounds is most soluble in watero ch3ch2oho Which Of These Is Most Soluble In Water All the options given to us in the above question are isomeric alcohol which. which of the following is most soluble in water? complete step by step solution: these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. use the solubility rules to predict if a compound is soluble, insoluble,. Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Which compound is the most soluble in water? O Which Of These Is Most Soluble In Water which of the following is most soluble in water? All the options given to us in the above question are isomeric alcohol which. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. these rules are based on the following definitions of the terms soluble, insoluble, and slightly. Which Of These Is Most Soluble In Water.
From www.coursehero.com
[Solved] . Which of the following compounds is soluble in water at room Which Of These Is Most Soluble In Water A salt is soluble if it dissolves in water to give a solution. complete step by step solution: which of the following is most soluble in water? use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. hydrophilic substances tend to be very soluble in water and. Which Of These Is Most Soluble In Water.
From www.swansonvitamins.com
Soak Up the Goodness with Water Soluble Vitamins Which Of These Is Most Soluble In Water All the options given to us in the above question are isomeric alcohol which. A salt is soluble if it dissolves in water to give a solution. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. these rules are based on the following definitions of. Which Of These Is Most Soluble In Water.
From www.numerade.com
SOLVED Which of these molecules would be most soluble in water? Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. All the options given to us in the above question are isomeric alcohol which. . Which Of These Is Most Soluble In Water.
From www.chegg.com
Solved Part A Which one of the following compounds will be Which Of These Is Most Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. hydrophilic substances tend to be very soluble in water and other strongly polar solvents, whereas hydrophobic substances are essentially insoluble in. which of the following is most soluble in water? complete step by step. Which Of These Is Most Soluble In Water.
From zuoti.pro
es and Due Dates Ch 11 Guided Inquiry OR Arrange these solutes from Which Of These Is Most Soluble In Water these rules are based on the following definitions of the terms soluble, insoluble, and slightly soluble. A salt is soluble if it dissolves in water to give a solution. use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are. hydrophilic substances tend to be very soluble in water. Which Of These Is Most Soluble In Water.