Heating Curve Of Water Practice Problems . The graph is not to scale but it is. Examine the heating curve of water and determine what is happening at each stage. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Examine the heating curve of h2o and determine what is happening at each stage. Evaporation of sweat requires energy and thus take excess heat away from the body. Some of the water that you drink may eventually be converted into. Answer the following using the above heating curve 1. What is the melting temperature of the above substance? Determine the energy required to convert 21.1. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. What is the energy needed to melt 326 grams of ice and heat it to 100°c? The graph is not to scale but it is.
from www.chegg.com
Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. The graph is not to scale but it is. What is the energy needed to melt 326 grams of ice and heat it to 100°c? What is the melting temperature of the above substance? Determine the energy required to convert 21.1. Evaporation of sweat requires energy and thus take excess heat away from the body. Examine the heating curve of water and determine what is happening at each stage. Answer the following using the above heating curve 1. Some of the water that you drink may eventually be converted into. Examine the heating curve of h2o and determine what is happening at each stage.
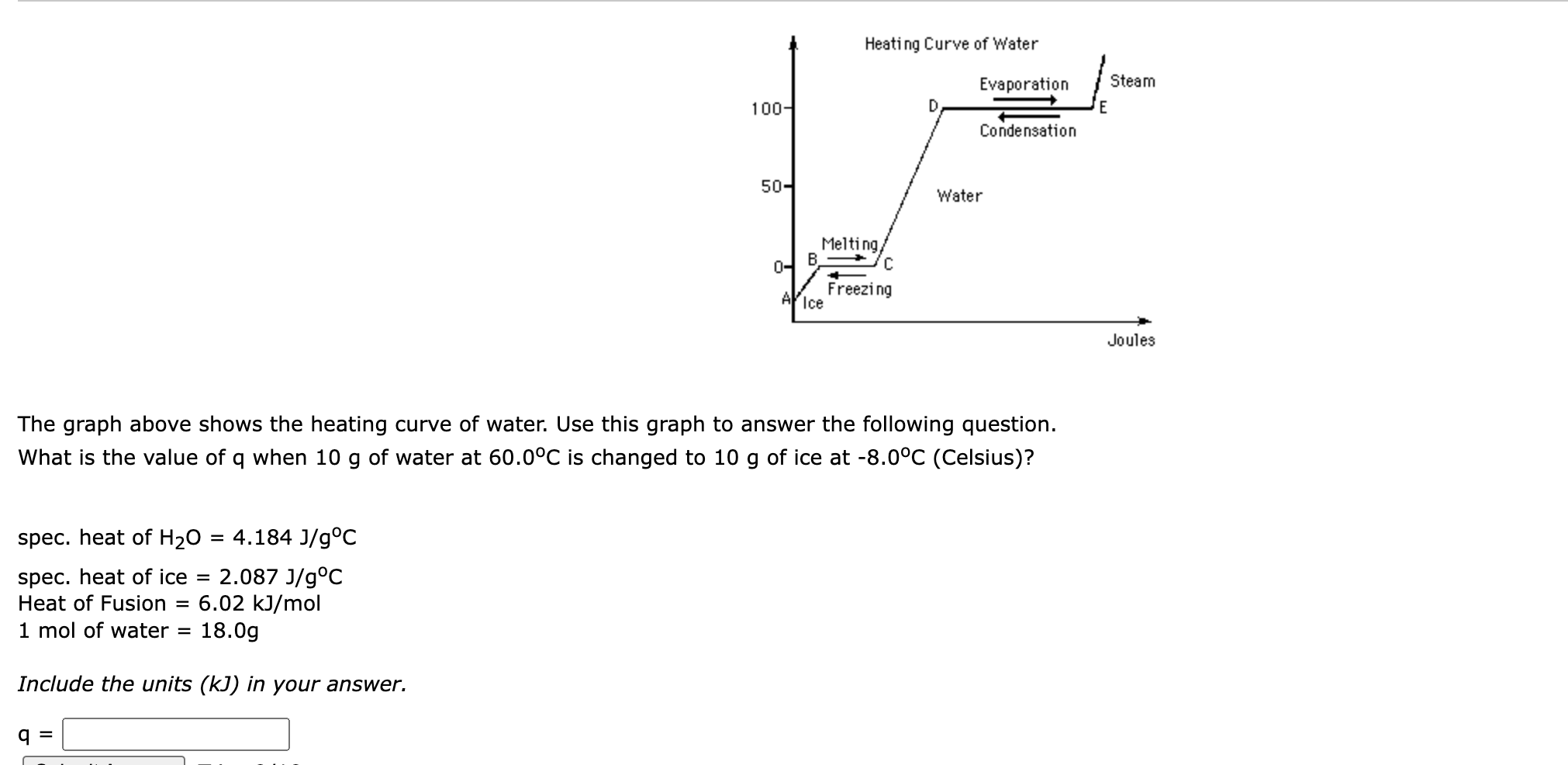
Solved The graph above shows the heating curve of water. Use
Heating Curve Of Water Practice Problems What is the melting temperature of the above substance? In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Determine the energy required to convert 21.1. Some of the water that you drink may eventually be converted into. Examine the heating curve of water and determine what is happening at each stage. The graph is not to scale but it is. Answer the following using the above heating curve 1. The graph is not to scale but it is. Examine the heating curve of h2o and determine what is happening at each stage. What is the melting temperature of the above substance? Evaporation of sweat requires energy and thus take excess heat away from the body. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. What is the energy needed to melt 326 grams of ice and heat it to 100°c?
From lessonfullantje.z19.web.core.windows.net
Draw And Label The Heating Curve For Water Heating Curve Of Water Practice Problems Answer the following using the above heating curve 1. Some of the water that you drink may eventually be converted into. Determine the energy required to convert 21.1. Examine the heating curve of water and determine what is happening at each stage. Examine the heating curve of h2o and determine what is happening at each stage. What is the melting. Heating Curve Of Water Practice Problems.
From www.scribd.com
Heating Curve of Water Worksheet Phase (Matter) Phase Transition Heating Curve Of Water Practice Problems In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. The graph is not to scale but it is. Examine the heating curve of h2o and determine what is happening at each stage. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. The graph is not to scale. Heating Curve Of Water Practice Problems.
From obropolox.blogspot.com
39 heating cooling curve calculations worksheet answers Worksheet Heating Curve Of Water Practice Problems Some of the water that you drink may eventually be converted into. Determine the energy required to convert 21.1. Examine the heating curve of water and determine what is happening at each stage. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Examine the heating curve of h2o and determine what is happening. Heating Curve Of Water Practice Problems.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve Of Water Practice Problems What is the melting temperature of the above substance? The graph is not to scale but it is. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Answer the following using the above heating curve 1. Evaporation of sweat requires energy and thus take excess heat away from the body. Examine the heating. Heating Curve Of Water Practice Problems.
From answermediacushing.z19.web.core.windows.net
Heating Curve Of Water Heating Curve Of Water Practice Problems What is the energy needed to melt 326 grams of ice and heat it to 100°c? In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Examine the heating curve of h2o and determine what is happening at each stage. Some of the water that you drink may eventually be converted into. The graph. Heating Curve Of Water Practice Problems.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Of Water Practice Problems Answer the following using the above heating curve 1. What is the melting temperature of the above substance? Examine the heating curve of water and determine what is happening at each stage. Determine the energy required to convert 21.1. The graph is not to scale but it is. In this simulation, students explore the heating curve for water from a. Heating Curve Of Water Practice Problems.
From amiahgokeestes.blogspot.com
Use the Heating Curve Below to Answer the Following Questions Heating Curve Of Water Practice Problems In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Examine the heating curve of h2o and determine what is happening at each stage. Heating curves show the phase changes that a substance undergoes as heat is continuously. Heating Curve Of Water Practice Problems.
From www.chegg.com
Solved The graph above shows the heating curve of water. Use Heating Curve Of Water Practice Problems Examine the heating curve of water and determine what is happening at each stage. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Some of the water that you drink may eventually be converted into. Determine the energy required to convert 21.1. Answer the following using the above heating curve 1. Evaporation of sweat. Heating Curve Of Water Practice Problems.
From wordworksheet.com
Heating And Cooling Curves Worksheet Heating Curve Of Water Practice Problems Examine the heating curve of h2o and determine what is happening at each stage. Some of the water that you drink may eventually be converted into. The graph is not to scale but it is. Examine the heating curve of water and determine what is happening at each stage. What is the energy needed to melt 326 grams of ice. Heating Curve Of Water Practice Problems.
From lessonlibnurselings.z21.web.core.windows.net
Heating Curve Of Water Explained Heating Curve Of Water Practice Problems The graph is not to scale but it is. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. What is the energy needed to melt 326 grams of ice and heat it to 100°c? What is the melting temperature of the above substance? The graph is not to scale but it is. Answer. Heating Curve Of Water Practice Problems.
From brainly.com
Consider the heating curve for water. A graph of the heating curve for Heating Curve Of Water Practice Problems The graph is not to scale but it is. The graph is not to scale but it is. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Determine the energy required to convert 21.1. Evaporation of sweat requires. Heating Curve Of Water Practice Problems.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Of Water Practice Problems Evaporation of sweat requires energy and thus take excess heat away from the body. Some of the water that you drink may eventually be converted into. Examine the heating curve of water and determine what is happening at each stage. The graph is not to scale but it is. In this simulation, students explore the heating curve for water from. Heating Curve Of Water Practice Problems.
From printableella99.z21.web.core.windows.net
Worksheet Heating Curve Of Water Heating Curve Of Water Practice Problems Evaporation of sweat requires energy and thus take excess heat away from the body. Some of the water that you drink may eventually be converted into. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. The graph is not to scale but it is. Examine the heating curve of water and determine what is. Heating Curve Of Water Practice Problems.
From studylib.net
Dougherty Valley HS AP Chemistry Name Heating Curve Practice Heating Curve Of Water Practice Problems Examine the heating curve of water and determine what is happening at each stage. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Determine the energy required to convert 21.1. What is the melting temperature of the above substance? Examine the heating curve of h2o and determine what is happening at each stage. Some. Heating Curve Of Water Practice Problems.
From diyworksheet.com
Worksheet Heating Curve Of Water Answers Heating Curve Of Water Practice Problems Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Determine the energy required to convert 21.1. Some of the water that you drink may eventually be converted into. Examine the heating curve of water and determine what is happening at each stage. Answer the following using the above heating curve 1. The graph is. Heating Curve Of Water Practice Problems.
From www.docsity.com
THE HEATING CURVE OF WATER Slides Chemistry Docsity Heating Curve Of Water Practice Problems The graph is not to scale but it is. Evaporation of sweat requires energy and thus take excess heat away from the body. The graph is not to scale but it is. Determine the energy required to convert 21.1. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Some of the water that. Heating Curve Of Water Practice Problems.
From www.pinterest.com
Heating curve calculation (benzene) Worksheets, Printable preschool Heating Curve Of Water Practice Problems Answer the following using the above heating curve 1. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Some of the water that you drink may eventually be converted into. Examine the heating curve of water and determine what is happening at each stage. The graph is not to scale but it is. In. Heating Curve Of Water Practice Problems.
From ar.inspiredpencil.com
Specific Heat Of Water Graph Heating Curve Of Water Practice Problems Some of the water that you drink may eventually be converted into. What is the melting temperature of the above substance? The graph is not to scale but it is. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Answer the following using the above heating curve 1. What is the energy needed to. Heating Curve Of Water Practice Problems.
From www.docsity.com
Heating Curve Worksheet 15 Questions Exercises Thermodynamics Docsity Heating Curve Of Water Practice Problems What is the energy needed to melt 326 grams of ice and heat it to 100°c? Answer the following using the above heating curve 1. Evaporation of sweat requires energy and thus take excess heat away from the body. Determine the energy required to convert 21.1. Examine the heating curve of water and determine what is happening at each stage.. Heating Curve Of Water Practice Problems.
From answerlistclapnets.z14.web.core.windows.net
Calculation Of Heating Curve For Water Heating Curve Of Water Practice Problems Answer the following using the above heating curve 1. What is the melting temperature of the above substance? Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. The graph is not to scale but it is. Evaporation of sweat requires energy and thus take excess heat away from the body. The graph is not. Heating Curve Of Water Practice Problems.
From ar.inspiredpencil.com
Graph Heating Curve For Water Heating Curve Of Water Practice Problems Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. Answer the following using the above heating curve 1. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Examine the heating curve of water and determine what is happening at each stage. Examine the heating curve of h2o. Heating Curve Of Water Practice Problems.
From www.chegg.com
Solved The graph above shows the heating curve of water. Use Heating Curve Of Water Practice Problems The graph is not to scale but it is. The graph is not to scale but it is. Determine the energy required to convert 21.1. Some of the water that you drink may eventually be converted into. Evaporation of sweat requires energy and thus take excess heat away from the body. Examine the heating curve of h2o and determine what. Heating Curve Of Water Practice Problems.
From studyschoolburman.z21.web.core.windows.net
Worksheet Heating Curve Of Water Heating Curve Of Water Practice Problems Examine the heating curve of h2o and determine what is happening at each stage. Determine the energy required to convert 21.1. Examine the heating curve of water and determine what is happening at each stage. The graph is not to scale but it is. The graph is not to scale but it is. Answer the following using the above heating. Heating Curve Of Water Practice Problems.
From quizzmediakrueger.z13.web.core.windows.net
Worksheets Heating Curve Of Water Heating Curve Of Water Practice Problems Evaporation of sweat requires energy and thus take excess heat away from the body. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. What is the melting temperature of the above substance? Determine the energy required to convert. Heating Curve Of Water Practice Problems.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Heating Curve Of Water Practice Problems Determine the energy required to convert 21.1. The graph is not to scale but it is. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Answer the following using the above heating curve 1. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Examine the heating. Heating Curve Of Water Practice Problems.
From www.madebyteachers.com
Heating Curve of Water Notes and Practice Made By Teachers Heating Curve Of Water Practice Problems What is the energy needed to melt 326 grams of ice and heat it to 100°c? Answer the following using the above heating curve 1. Evaporation of sweat requires energy and thus take excess heat away from the body. What is the melting temperature of the above substance? The graph is not to scale but it is. The graph is. Heating Curve Of Water Practice Problems.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Of Water Practice Problems Evaporation of sweat requires energy and thus take excess heat away from the body. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Examine the heating curve of h2o and determine what is happening at each stage. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. The. Heating Curve Of Water Practice Problems.
From www.owhentheyanks.com
Heating And Cooling Curve Worksheet Heating Curve Of Water Practice Problems The graph is not to scale but it is. Determine the energy required to convert 21.1. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Examine the heating curve of h2o and determine what is happening at each stage. What is the energy needed to melt 326 grams of ice and heat it. Heating Curve Of Water Practice Problems.
From dxoxfozzn.blob.core.windows.net
Heating Curve Of Water Experiment Grade 10 at Roberto Cole blog Heating Curve Of Water Practice Problems Evaporation of sweat requires energy and thus take excess heat away from the body. Some of the water that you drink may eventually be converted into. The graph is not to scale but it is. Examine the heating curve of water and determine what is happening at each stage. The graph is not to scale but it is. In this. Heating Curve Of Water Practice Problems.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Heating Curve Of Water Practice Problems What is the melting temperature of the above substance? The graph is not to scale but it is. Evaporation of sweat requires energy and thus take excess heat away from the body. Examine the heating curve of h2o and determine what is happening at each stage. Examine the heating curve of water and determine what is happening at each stage.. Heating Curve Of Water Practice Problems.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve Of Water Practice Problems The graph is not to scale but it is. Answer the following using the above heating curve 1. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Some of the water that you drink may eventually be converted into. Heating curves show the phase changes that a substance undergoes as heat is continuously. Heating Curve Of Water Practice Problems.
From printablelibagnames.z13.web.core.windows.net
Heating Curve Of Water Explained Heating Curve Of Water Practice Problems What is the melting temperature of the above substance? Evaporation of sweat requires energy and thus take excess heat away from the body. What is the energy needed to melt 326 grams of ice and heat it to 100°c? Some of the water that you drink may eventually be converted into. Determine the energy required to convert 21.1. Answer the. Heating Curve Of Water Practice Problems.
From printablelibmolines.z13.web.core.windows.net
Heating Curve Of Water Worksheet Heating Curve Of Water Practice Problems Determine the energy required to convert 21.1. Evaporation of sweat requires energy and thus take excess heat away from the body. Some of the water that you drink may eventually be converted into. Examine the heating curve of water and determine what is happening at each stage. What is the energy needed to melt 326 grams of ice and heat. Heating Curve Of Water Practice Problems.
From printablemagicbishop.z21.web.core.windows.net
Worksheets Heating Curve Of Water Heating Curve Of Water Practice Problems Determine the energy required to convert 21.1. The graph is not to scale but it is. The graph is not to scale but it is. Evaporation of sweat requires energy and thus take excess heat away from the body. Answer the following using the above heating curve 1. Some of the water that you drink may eventually be converted into.. Heating Curve Of Water Practice Problems.
From worksheetdbyrent.z19.web.core.windows.net
Heat Curve Of Water Heating Curve Of Water Practice Problems Answer the following using the above heating curve 1. Heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. What is the melting temperature of the above substance? The graph is not to scale but it is. Examine the heating curve of water and determine what is happening at each stage. In this simulation, students. Heating Curve Of Water Practice Problems.