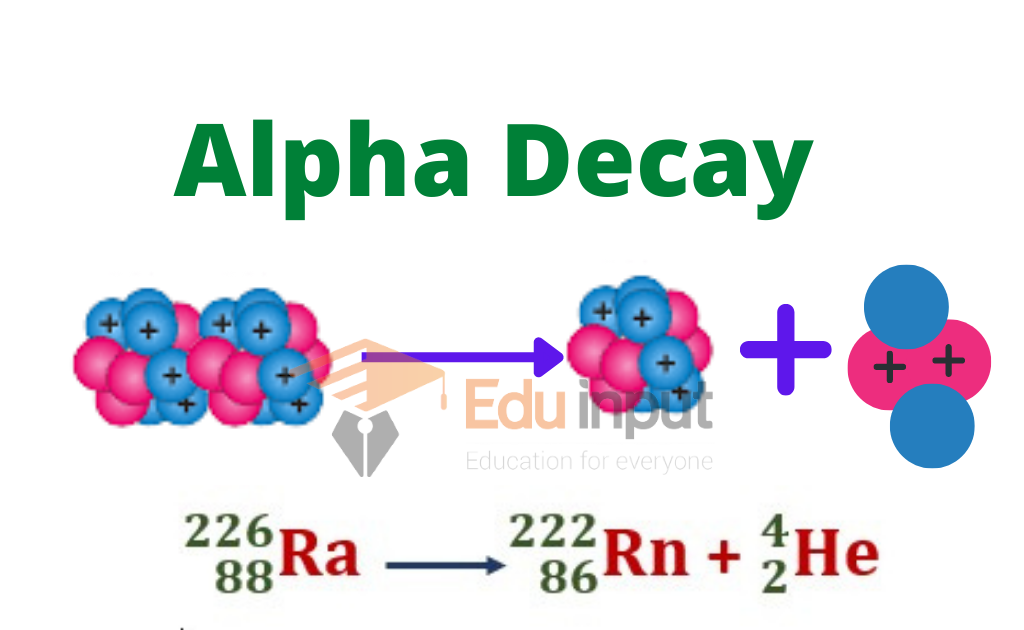
Radioactive Decay Alpha . An example of a nucleus that undergoes alpha decay. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The nuclear disintegration process that emits alpha particles is called alpha decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic.
from eduinput.com
An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. An example of a nucleus that undergoes alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. The nuclear disintegration process that emits alpha particles is called alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic.
Nuclear Transmutation Decay Reactions, Alpha Decay, Beta Decay, and
Radioactive Decay Alpha Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The nuclear disintegration process that emits alpha particles is called alpha decay. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An example of a nucleus that undergoes alpha decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha.
From www.slideserve.com
PPT Nuclear Chemistry PowerPoint Presentation, free download ID1537751 Radioactive Decay Alpha An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. The nuclear disintegration process that emits. Radioactive Decay Alpha.
From www.slideserve.com
PPT Alpha Decay PowerPoint Presentation, free download ID242969 Radioactive Decay Alpha Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. An example of a nucleus that undergoes alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The nuclear disintegration process that emits. Radioactive Decay Alpha.
From www.showme.com
Alpha decay problem Chemistry, Radioactivity, Science, Nuclear Radioactive Decay Alpha Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. An example of a nucleus that undergoes alpha decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The nuclear disintegration process that emits alpha particles is called alpha decay. Radioactive decay is the process in which. Radioactive Decay Alpha.
From sciencedrill.com
A Guide to Alpha Decay Including Equations and Uses Science Drill Radioactive Decay Alpha Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Radioactive decay is the process. Radioactive Decay Alpha.
From shakerscience.weebly.com
Unit 11 Nuclear Chemistry Physical Science Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. An example of a nucleus that undergoes alpha decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Radioactive decay is the. Radioactive Decay Alpha.
From www.coolkidfacts.com
Radioactivity and Radiation Cool Kid Facts Radioactive Decay Alpha Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The nuclear disintegration process that emits alpha particles is called alpha decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by. Radioactive Decay Alpha.
From www.alamy.com
Radioactive decay diagram hires stock photography and images Alamy Radioactive Decay Alpha Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. The nuclear disintegration process that emits alpha particles is called alpha decay. Radioactive decay is the process in which. Radioactive Decay Alpha.
From eduinput.com
Nuclear Transmutation Decay Reactions, Alpha Decay, Beta Decay, and Radioactive Decay Alpha Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The most common types of radioactivity are α decay, β decay, γ emission, positron emission,. Radioactive Decay Alpha.
From www.slideserve.com
PPT Radioactivity PowerPoint Presentation, free download ID2485771 Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An example of a nucleus that undergoes alpha decay. The nuclear disintegration process that emits alpha particles is called alpha decay. The most common types of radioactivity. Radioactive Decay Alpha.
From www.youtube.com
Different types of decay Alpha vs. Beta vs. Gamma decay Visual Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles. Radioactive Decay Alpha.
From studiousguy.com
7 Radioactive Decay Examples in Real Life StudiousGuy Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. An example of a nucleus that undergoes alpha decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Alpha decay,. Radioactive Decay Alpha.
From www.ck12.org
Alpha Decay CK12 Foundation Radioactive Decay Alpha The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. The nuclear disintegration process that emits alpha particles is called alpha decay. An example of a nucleus that undergoes alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. Nuclei. Radioactive Decay Alpha.
From www.scienceabc.com
Why Is The Term "HalfLife" Used To Measure Radioactivity? » ScienceABC Radioactive Decay Alpha An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The nuclear disintegration process that emits alpha particles is called alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. The most common types of radioactivity are α decay, β decay, γ emission,. Radioactive Decay Alpha.
From www.nuclear-power.com
Radioactive Decay Modes Radioactive Decay Alpha An example of a nucleus that undergoes alpha decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay.. Radioactive Decay Alpha.
From www.slideshare.net
Radioactive decay Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An alpha particle is a positively charged particle consisting of two protons and two neutrons,. Radioactive Decay Alpha.
From a15.beauty
Radioactive Decay Alpha Beta Gamma Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha. Radioactive Decay Alpha.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Radioactive Decay Alpha Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay.. Radioactive Decay Alpha.
From www.youtube.com
Radioactive Decay/Nuclear Radiation (Alpha, Beta, Gamma) GCSE Physics Radioactive Decay Alpha The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Radioactive decay is the process in which an unstable nucleus. Radioactive Decay Alpha.
From sciencedrill.com
Calculating and Alpha Decay Energy or QValue Science Drill Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Alpha decay, the emission of helium. Radioactive Decay Alpha.
From www.rankred.com
What is Alpha Decay? Definition Equation Example RankRed Radioactive Decay Alpha Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The most common types of radioactivity are α. Radioactive Decay Alpha.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Radioactive Decay Alpha An example of a nucleus that undergoes alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. The nuclear disintegration process that emits alpha particles is. Radioactive Decay Alpha.
From www.sliderbase.com
Radioactive Decay, Nuclear Reactions Presentation Physics Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An example of a nucleus that undergoes alpha decay. Radioactive decay is the. Radioactive Decay Alpha.
From en.wikipedia.org
Alpha particle Wikipedia Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. Alpha decay, the emission of helium ions, exhibits sharp. Radioactive Decay Alpha.
From www.youtube.com
Radioactive decaysalpha decay YouTube Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. An example of a nucleus that undergoes alpha decay. An alpha particle is a positively charged particle consisting of two protons and. Radioactive Decay Alpha.
From modernphysicsls.blogspot.com
Radioactivity & Alpha Decay. Radioactive Decay Alpha Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. An example of a nucleus that undergoes alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An alpha particle is a positively charged particle consisting of two protons. Radioactive Decay Alpha.
From www.slideserve.com
PPT Review Alpha Decay PowerPoint Presentation, free download ID Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. An example of a nucleus that undergoes alpha decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The most common types of radioactivity are α decay,. Radioactive Decay Alpha.
From sciencenotes.org
Radioactivity and the Types of Radioactive Decay Radioactive Decay Alpha The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An example of a nucleus that undergoes alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha.. Radioactive Decay Alpha.
From www.slideserve.com
PPT Nuclear Decays PowerPoint Presentation, free download ID309465 Radioactive Decay Alpha An example of a nucleus that undergoes alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. The nuclear disintegration process that emits alpha particles is called alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. The most common types of radioactivity. Radioactive Decay Alpha.
From pixels.com
Simulation Of Radioactive Alpha Decay Photograph by Prof. K.seddon & Dr Radioactive Decay Alpha An example of a nucleus that undergoes alpha decay. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Radioactive. Radioactive Decay Alpha.
From www.slideserve.com
PPT DISCOVERERS OF RADIOACTIVITY PowerPoint Presentation ID6535776 Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha decay, the. Radioactive Decay Alpha.
From www.simply.science
Alpha Decay Radioactive Decay Alpha An alpha particle is a positively charged particle consisting of two protons and two neutrons, identical to the nucleus of a helium. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an. Radioactive Decay Alpha.
From www.shalom-education.com
Nuclear Decay Equations GCSE Physics Revision Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. An example of a nucleus that undergoes alpha decay. Alpha decay, the emission of helium ions, exhibits sharp line spectra when. Radioactive Decay Alpha.
From eduinput.com
Nuclear Transmutation Decay Reactions, Alpha Decay, Beta Decay, and Radioactive Decay Alpha The nuclear disintegration process that emits alpha particles is called alpha decay. Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. Alpha decay, the emission of helium ions, exhibits sharp line spectra when spectroscopic. An alpha particle is a positively charged particle consisting of two protons and two neutrons,. Radioactive Decay Alpha.
From www.slideserve.com
PPT Radioactivity PowerPoint Presentation, free download ID2485771 Radioactive Decay Alpha The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. An example of a nucleus that undergoes alpha decay. Radioactive decay is the process in which an unstable nucleus spontaneously loses energy by emitting ionizing particles and radiation. An alpha particle is a positively charged particle consisting of two protons and two. Radioactive Decay Alpha.
From www.youtube.com
RADIOACTIVITY. Decay modes. Nuclear disintegration alpha beta gamma α Radioactive Decay Alpha Alpha decay, type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. An example of a nucleus that undergoes alpha decay. Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. Alpha decay, the. Radioactive Decay Alpha.