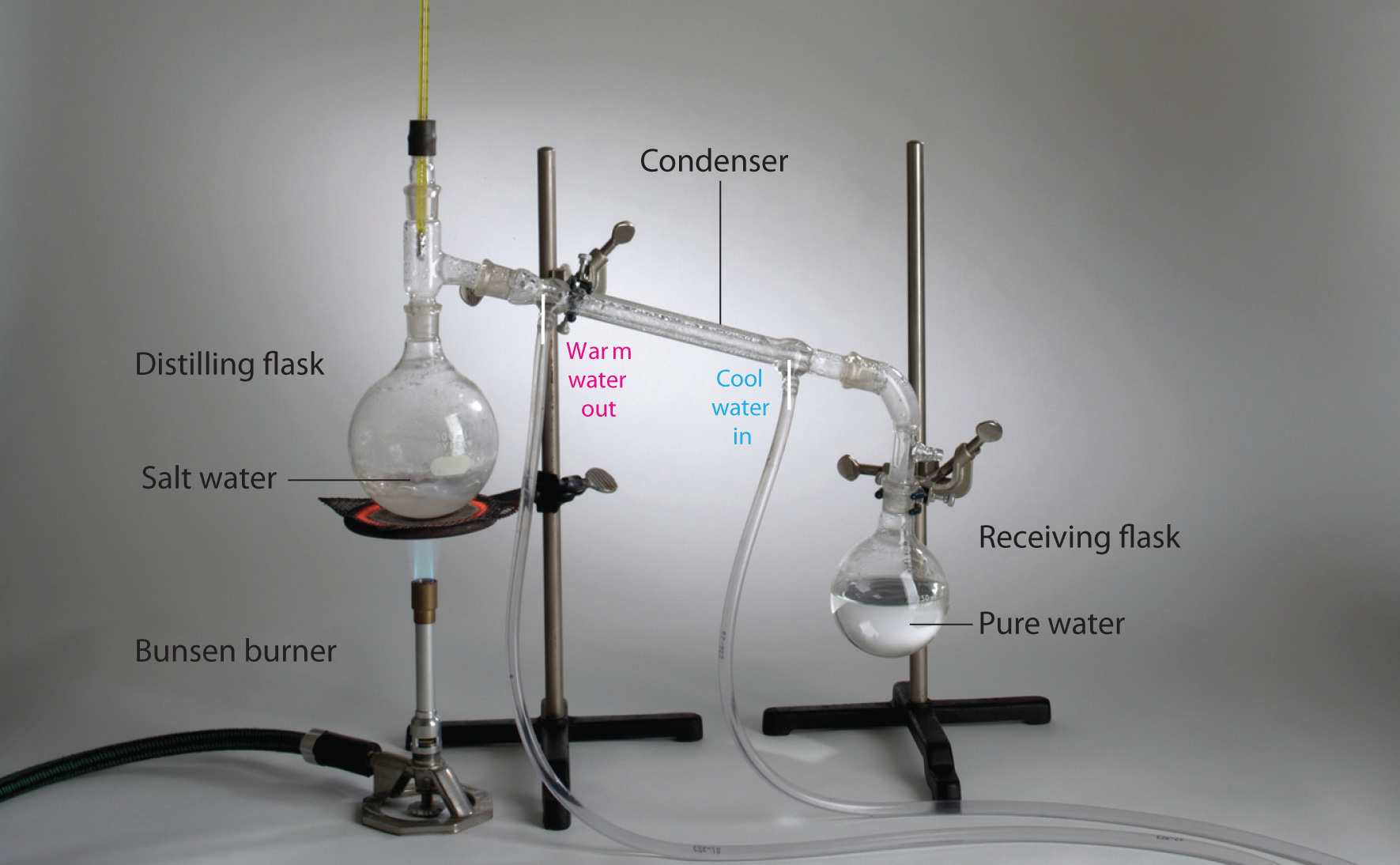
Why Is Boiling Point Important In Distillation . A change in boiling point during distillation is an indication of impurity. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. As the liquid being distilled is. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. However, it illustrates an important principle that is. A pure liquid has a constant boiling point (at constant pressure). Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere.
from chem.libretexts.org
Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. A pure liquid has a constant boiling point (at constant pressure). However, it illustrates an important principle that is. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. A change in boiling point during distillation is an indication of impurity. Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation.
1A.3 Classifying Matter Chemistry LibreTexts
Why Is Boiling Point Important In Distillation Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. As the liquid being distilled is. A change in boiling point during distillation is an indication of impurity. However, it illustrates an important principle that is. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. A pure liquid has a constant boiling point (at constant pressure). Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Why Is Boiling Point Important In Distillation A change in boiling point during distillation is an indication of impurity. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. A pure liquid has a constant. Why Is Boiling Point Important In Distillation.
From cetebqnt.blob.core.windows.net
Does Distilled Water Boil at Joshua Fisher blog Why Is Boiling Point Important In Distillation Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. A change in boiling point during distillation is an indication of impurity. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Elevation of the boiling. Why Is Boiling Point Important In Distillation.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and Why Is Boiling Point Important In Distillation Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. Elevation of the boiling point with an increase in external pressure, while. Why Is Boiling Point Important In Distillation.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Is Boiling Point Important In Distillation Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Distillation columns use a thermal process that involves heating and cooling. Why Is Boiling Point Important In Distillation.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Why Is Boiling Point Important In Distillation Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. A change in boiling point during distillation is an indication of impurity. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting. Why Is Boiling Point Important In Distillation.
From slidetodoc.com
Properties of matter Pure Substances Mixtures Solutions Separation Why Is Boiling Point Important In Distillation However, it illustrates an important principle that is. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. In the simplest terms, a distillation involves boiling. Why Is Boiling Point Important In Distillation.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Why Is Boiling Point Important In Distillation Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Simple distillation in the lab is generally appropriate for separating out. Why Is Boiling Point Important In Distillation.
From chem.libretexts.org
Fractional Distillation of Ideal Mixtures Chemistry LibreTexts Why Is Boiling Point Important In Distillation Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. A change in boiling point during distillation is an indication of impurity. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at. Why Is Boiling Point Important In Distillation.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Why Is Boiling Point Important In Distillation Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. A pure liquid has. Why Is Boiling Point Important In Distillation.
From guidemanualeruptivity.z14.web.core.windows.net
Boiling Point Composition Diagram Why Is Boiling Point Important In Distillation Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. A pure liquid has a constant boiling point (at constant pressure). However, it illustrates an important principle that is. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Elevation of. Why Is Boiling Point Important In Distillation.
From www.geeksforgeeks.org
Distillation Definition, Meaning, Principle, Types & Uses Why Is Boiling Point Important In Distillation A pure liquid has a constant boiling point (at constant pressure). A change in boiling point during distillation is an indication of impurity. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. However, it illustrates an important principle that is. Distillation columns use a thermal process that involves heating. Why Is Boiling Point Important In Distillation.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube Why Is Boiling Point Important In Distillation A change in boiling point during distillation is an indication of impurity. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into.. Why Is Boiling Point Important In Distillation.
From future4200.com
Understanding vacuum and boiling point relationship Distillation Why Is Boiling Point Important In Distillation A pure liquid has a constant boiling point (at constant pressure). As the liquid being distilled is. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important. Why Is Boiling Point Important In Distillation.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Why Is Boiling Point Important In Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point. Why Is Boiling Point Important In Distillation.
From mammothmemory.net
Fractional distillation is the separation of substances Why Is Boiling Point Important In Distillation A change in boiling point during distillation is an indication of impurity. A pure liquid has a constant boiling point (at constant pressure). As the liquid being distilled is. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Distillation columns use a thermal process that. Why Is Boiling Point Important In Distillation.
From www.studypool.com
SOLUTION Boiling point and distillation Studypool Why Is Boiling Point Important In Distillation Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. However, it illustrates an important principle that is. A pure liquid has a constant boiling point (at constant pressure). The temperature. Why Is Boiling Point Important In Distillation.
From amerlab.com
Sub Boiling Distillation Systems Sub Boiling Distillation Benefits Why Is Boiling Point Important In Distillation A change in boiling point during distillation is an indication of impurity. Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. Why Is Boiling Point Important In Distillation.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog Why Is Boiling Point Important In Distillation Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. A change in boiling point during distillation is an indication of impurity. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through. Why Is Boiling Point Important In Distillation.
From www.youtube.com
BOILING POINT EQUILIBRIUM DIAGRAM DIAGRAM Why Is Boiling Point Important In Distillation A change in boiling point during distillation is an indication of impurity. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0. Why Is Boiling Point Important In Distillation.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Why Is Boiling Point Important In Distillation Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. A change in boiling point during distillation is an indication of impurity. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0. Why Is Boiling Point Important In Distillation.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Why Is Boiling Point Important In Distillation However, it illustrates an important principle that is. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. As. Why Is Boiling Point Important In Distillation.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective Why Is Boiling Point Important In Distillation Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through. Why Is Boiling Point Important In Distillation.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Why Is Boiling Point Important In Distillation A pure liquid has a constant boiling point (at constant pressure). Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. Because different compounds often have different boiling points, the components often separate from a mixture when the. Why Is Boiling Point Important In Distillation.
From www.goodscience.com.au
Elements, Compounds and Mixtures Good Science Why Is Boiling Point Important In Distillation Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. A change in boiling point during distillation is an indication of impurity. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids,. Why Is Boiling Point Important In Distillation.
From mungfali.com
Phase Diagram Boiling Point Why Is Boiling Point Important In Distillation The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. As the liquid being distilled is. However, it illustrates an important principle that is. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling. Why Is Boiling Point Important In Distillation.
From www.numerade.com
SOLVED Define the term "boiling point" and describe what is happening Why Is Boiling Point Important In Distillation A pure liquid has a constant boiling point (at constant pressure). Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Simple distillation (the procedure outlined below) can be used effectively. Why Is Boiling Point Important In Distillation.
From www.pinterest.com
Distillation is a procedure that separates a mixture of liquids with Why Is Boiling Point Important In Distillation The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling. Why Is Boiling Point Important In Distillation.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting Why Is Boiling Point Important In Distillation The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. Simple distillation in the lab is generally appropriate for separating out a molecular. Why Is Boiling Point Important In Distillation.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Why Is Boiling Point Important In Distillation Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or liquids, into. A pure liquid has a constant boiling point (at constant pressure). In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Because different compounds often have different. Why Is Boiling Point Important In Distillation.
From www.yumpu.com
5. Distillation and Boiling Points Why Is Boiling Point Important In Distillation Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. Because different compounds often have different boiling points, the components often separate. Why Is Boiling Point Important In Distillation.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Why Is Boiling Point Important In Distillation Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through. Why Is Boiling Point Important In Distillation.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Why Is Boiling Point Important In Distillation Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled. Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. Simple distillation in the lab is generally appropriate for separating out a molecular substance with. Why Is Boiling Point Important In Distillation.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Why Is Boiling Point Important In Distillation The temperature reading is important, because, at normal conditions, the temperature of the vapor passing through is the same as the boiling point. As the liquid being distilled is. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Simple distillation in the lab is generally appropriate for separating out a. Why Is Boiling Point Important In Distillation.
From www.worksheetsplanet.com
What is Boiling Point Why Is Boiling Point Important In Distillation Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. However, it illustrates an important principle that is. A change in boiling point during distillation is an indication of impurity. As the liquid being distilled is. In the simplest terms, a distillation involves boiling a. Why Is Boiling Point Important In Distillation.
From slideplayer.com
Fractional Distillation ppt download Why Is Boiling Point Important In Distillation Elevation of the boiling point with an increase in external pressure, while important in cooking and sterilizing food or utensils, is less important in distillation. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘ c and ∼ 2 0 0 ∘ c, as. A change in. Why Is Boiling Point Important In Distillation.