Iron + Oxygen Yields Iron (Iii) Oxide . Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o 3 is +3. In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced equation represents the ratio of reactants to products. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The iron reacts with the oxygen to produce. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here).
from stock.adobe.com
When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced equation represents the ratio of reactants to products. The oxidation state of fe 2 o 3 is +3. The iron reacts with the oxygen to produce. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here).
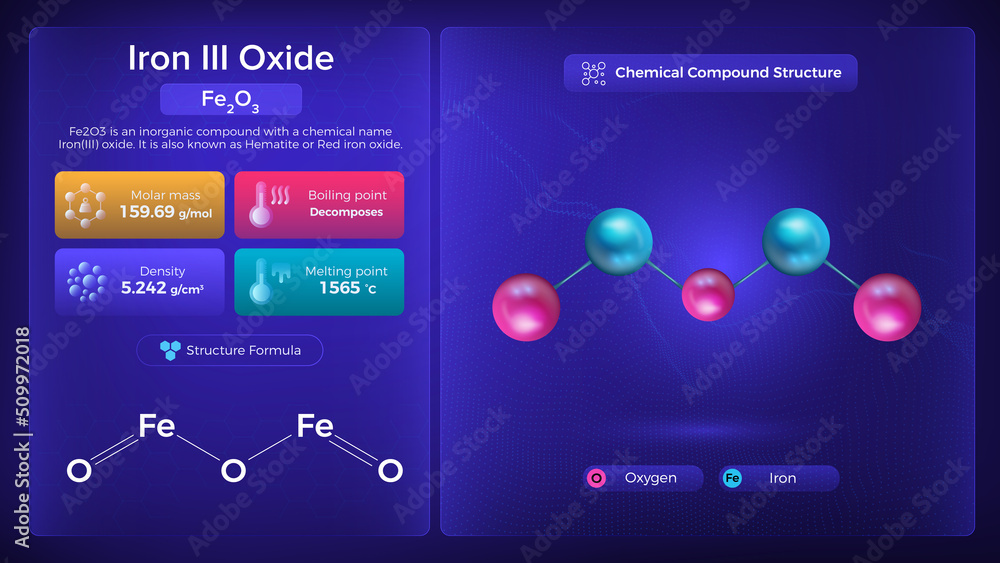
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector
Iron + Oxygen Yields Iron (Iii) Oxide The iron reacts with the oxygen to produce. The stoichiometry of a balanced equation represents the ratio of reactants to products. The oxidation state of fe 2 o 3 is +3. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. In our example of forming iron(iii) oxide from iron. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here).
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. In our example of forming iron(iii) oxide from iron. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here).. Iron + Oxygen Yields Iron (Iii) Oxide.
From slideplayer.com
Chemical Reactions and Stoichiometry ppt download Iron + Oxygen Yields Iron (Iii) Oxide The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. The stoichiometry of a balanced equation represents the ratio of reactants to products. When two smaller substances combine to. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The stoichiometry of a balanced equation represents the ratio of reactants to products. When two smaller substances combine to form only one product it is usually a. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o 3 is +3. The iron reacts with the oxygen to produce. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The stoichiometry of a balanced equation represents the ratio. Iron + Oxygen Yields Iron (Iii) Oxide.
From mungfali.com
Iron Oxide Phase Diagram Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. The oxidation state of fe 2 o 3 is +3. The iron reacts with the oxygen to produce. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Fe 2. Iron + Oxygen Yields Iron (Iii) Oxide.
From stock.adobe.com
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron + Oxygen Yields Iron (Iii) Oxide When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. In our example of forming iron(iii) oxide from iron. The reaction of iron and. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.slideserve.com
PPT The Law of Conservation of Matter PowerPoint Presentation, free Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The oxidation state of fe 2 o 3 is +3. When two smaller substances combine to form only one product it is usually a synthesis, or combination. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. The stoichiometry of a balanced equation represents the ratio of reactants to products. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron + Oxygen Yields Iron (Iii) Oxide The iron reacts with the oxygen to produce. The stoichiometry of a balanced equation represents the ratio of reactants to products. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The oxidation state of fe 2. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide Iron + Oxygen Yields Iron (Iii) Oxide The oxidation state of fe 2 o 3 is +3. In our example of forming iron(iii) oxide from iron. The iron reacts with the oxygen to produce. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here).. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVED For the following reaction, 8 5 . 0 grams of iron are allowed Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction.. Iron + Oxygen Yields Iron (Iii) Oxide.
From slideplayer.com
Chemical Reactions and Stoichiometry ppt download Iron + Oxygen Yields Iron (Iii) Oxide The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). In our example of forming iron(iii) oxide from iron. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms.. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.researchgate.net
Ironoxygen phase diagram [17]. Download Scientific Diagram Iron + Oxygen Yields Iron (Iii) Oxide The stoichiometry of a balanced equation represents the ratio of reactants to products. In our example of forming iron(iii) oxide from iron. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.geologypage.com
Discovery of new iron oxides points to large oxygen source inside the Iron + Oxygen Yields Iron (Iii) Oxide The oxidation state of fe 2 o 3 is +3. In our example of forming iron(iii) oxide from iron. The iron reacts with the oxygen to produce. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. When two smaller substances combine to form only one product it is usually. Iron + Oxygen Yields Iron (Iii) Oxide.
From slideplayer.com
Chemical Reactions. ppt download Iron + Oxygen Yields Iron (Iii) Oxide The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). In our example of forming iron(iii) oxide from iron. The iron reacts with the oxygen to produce. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. When two smaller substances combine to form only one product. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.researchgate.net
The iron/iron oxide phase diagram, also known as the BaurGleassner Iron + Oxygen Yields Iron (Iii) Oxide The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). In our example of forming iron(iii) oxide from iron. The oxidation state of fe 2 o 3 is +3. The stoichiometry of a balanced equation represents the ratio of reactants to products. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.chegg.com
Solved Iron reacts with oxygen at high temperatures to form Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The stoichiometry of a balanced equation represents the ratio of reactants to products. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). In our example of forming iron(iii) oxide from iron. The iron reacts with the. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron + Oxygen Yields Iron (Iii) Oxide The oxidation state of fe 2 o 3 is +3. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. In our example of forming iron(iii) oxide from iron. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). Fe 2 o 3 is the chemical formula of iron(iii). Iron + Oxygen Yields Iron (Iii) Oxide.
From ceramics.org
Materials for 6G technology Scientists refine synthesis of rare iron Iron + Oxygen Yields Iron (Iii) Oxide The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced equation represents the ratio of reactants to products. The iron reacts with the oxygen to produce. When two smaller substances combine to form only one product it is usually a synthesis, or combination. Iron + Oxygen Yields Iron (Iii) Oxide.
From xaydungso.vn
Iron 3 Oxide Tính Chất, Cấu Trúc và Ứng Dụng Hàng Đầu Iron + Oxygen Yields Iron (Iii) Oxide The oxidation state of fe 2 o 3 is +3. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. When two smaller substances combine to form only one product it is usually a synthesis, or combination. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.youtube.com
when iron rusts, solid iron reacts with gaseous oxygen to form solid Iron + Oxygen Yields Iron (Iii) Oxide The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced equation represents the ratio of reactants to products.. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVED When iron rusts, solid iron reacts with gaseous oxygen to form Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.hithaonthego.com
Iron Oxide Hitha On The Go Iron + Oxygen Yields Iron (Iii) Oxide When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The iron reacts with the oxygen to produce. In our example of forming iron(iii) oxide from iron. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The stoichiometry of a balanced equation represents the ratio of reactants to. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron(iii). Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron + Oxygen Yields Iron (Iii) Oxide When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced equation represents the ratio of reactants to products.. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVED Calculate Analysis of a compound composed of iron and oxygen Iron + Oxygen Yields Iron (Iii) Oxide When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The stoichiometry of a balanced equation represents the ratio of reactants to products. In our example of forming iron(iii) oxide from iron. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms.. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.chegg.com
Solved Iron reacts with oxygen to produce iron(III) oxide. Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The iron reacts with the oxygen to produce. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. When two smaller substances combine to form only one product. Iron + Oxygen Yields Iron (Iii) Oxide.
From fyoqutlma.blob.core.windows.net
What Does Iron (Iii) Oxide Mean at Anthony Silvernail blog Iron + Oxygen Yields Iron (Iii) Oxide The iron reacts with the oxygen to produce. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The stoichiometry of a balanced equation represents the ratio of reactants to products. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). In our example of forming iron(iii) oxide from. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
⏩SOLVEDIron reacts with oxygen as shown. 4 Fe(s)+3 O2(g) → 2… Numerade Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. The stoichiometry of a balanced equation represents the ratio of reactants to products. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o 3 is +3. When two smaller substances combine to form only. Iron + Oxygen Yields Iron (Iii) Oxide.
From mungfali.com
Iron Oxide Phase Diagram Iron + Oxygen Yields Iron (Iii) Oxide When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The stoichiometry of a balanced equation represents the ratio of reactants to products. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. In our example of forming iron(iii) oxide from iron.. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.numerade.com
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a Iron + Oxygen Yields Iron (Iii) Oxide In our example of forming iron(iii) oxide from iron. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The stoichiometry of a balanced equation represents the ratio of reactants to products. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The iron reacts with the oxygen to produce. The oxidation state of fe 2 o 3 is +3. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). When two smaller substances combine to form only one. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.solutionspile.com
[Solved] Under certain conditions, the substances iron and Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. When two smaller substances combine to form only one product it is usually a synthesis, or combination reaction. The reaction of iron and oxygen yields iron(iii) oxide (other reactions are here). The oxidation state of fe 2 o 3 is. Iron + Oxygen Yields Iron (Iii) Oxide.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) YouTube Iron + Oxygen Yields Iron (Iii) Oxide Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. In our example of forming iron(iii) oxide from iron. The oxidation state of fe 2 o 3 is +3. The stoichiometry of a balanced equation represents the ratio of reactants to products. The reaction of iron and oxygen yields iron(iii). Iron + Oxygen Yields Iron (Iii) Oxide.