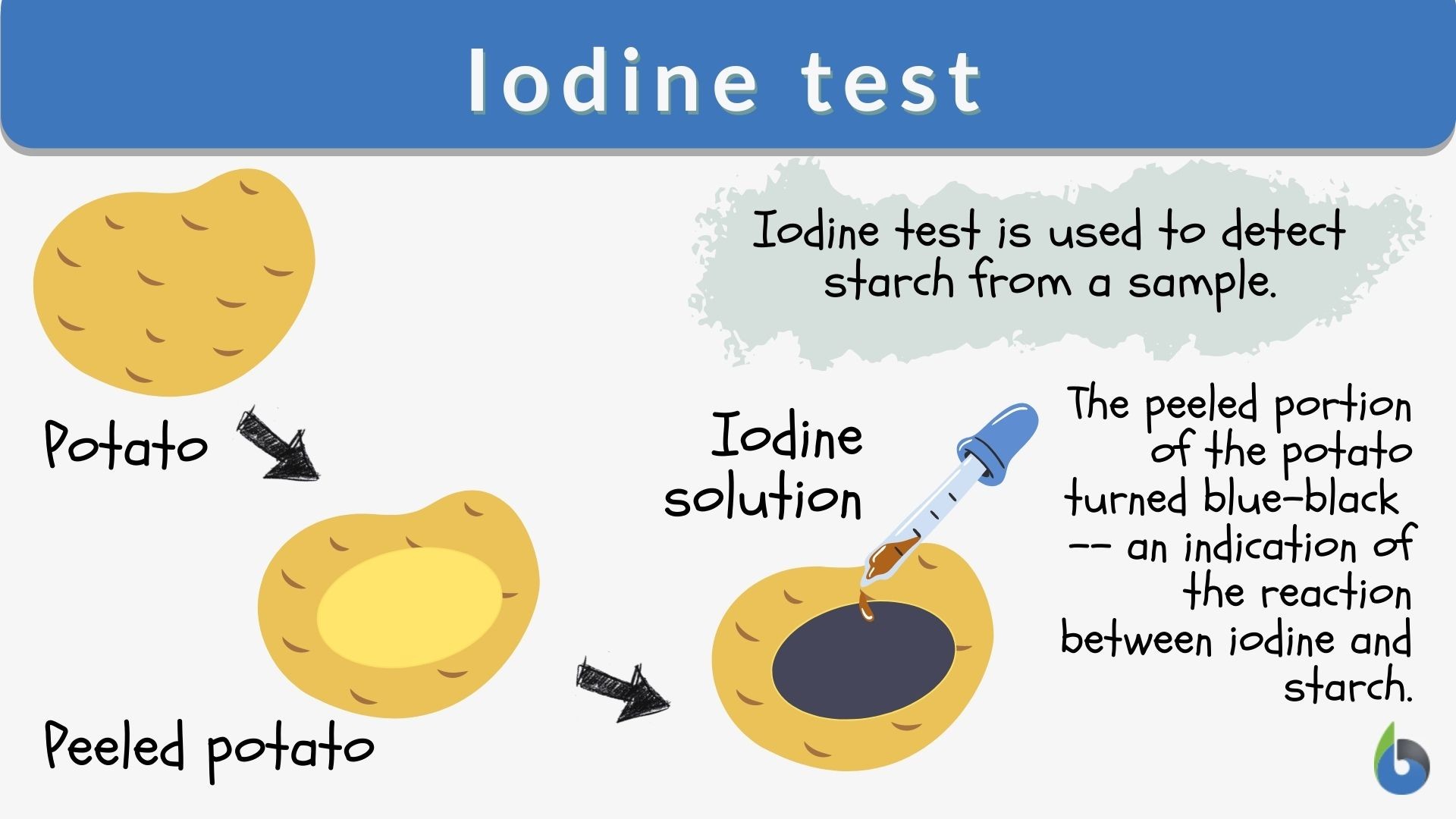
Starch Indicator Chemical Reaction . Since then, many other investigators have added to. This test has a variation. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. They produced the strikingly colorful demonstration by adding starch indicator. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. One molecule of vitamin c is needed to.
from www.animalia-life.club
When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. This test has a variation. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. One molecule of vitamin c is needed to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Since then, many other investigators have added to. They produced the strikingly colorful demonstration by adding starch indicator.
Starch Amylase Reaction Equation
Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. One molecule of vitamin c is needed to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. They produced the strikingly colorful demonstration by adding starch indicator. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. Since then, many other investigators have added to. This test has a variation.
From www.biologyonline.com
Iodine test Definition and Examples Biology Online Dictionary Starch Indicator Chemical Reaction Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. This test has a variation. They produced the strikingly colorful demonstration by adding starch indicator. One molecule of vitamin c is needed. Starch Indicator Chemical Reaction.
From www.fishersci.co.uk
Starch indicator solution 1, Acculute Standard Volumetric Solution Starch Indicator Chemical Reaction Since then, many other investigators have added to. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. They produced the strikingly colorful demonstration by adding starch indicator. Iodometric titration is a method used to measure. Starch Indicator Chemical Reaction.
From www.chegg.com
Solved 4. Using starch solution as an indicator, the iodine Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Since then, many other investigators have added to. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. This test has a variation. They produced the strikingly colorful demonstration by. Starch Indicator Chemical Reaction.
From www.fishersci.com
Starch Indicator, 1 (w/v) Aqueous Solution, Stabilized, Spectrum Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. They produced the strikingly colorful demonstration by adding starch indicator. Since then, many other investigators have added to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. One molecule of vitamin c is. Starch Indicator Chemical Reaction.
From www.animalia-life.club
Starch Amylase Reaction Equation Starch Indicator Chemical Reaction Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. They produced the strikingly colorful demonstration by adding starch indicator. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an. Starch Indicator Chemical Reaction.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Starch Indicator Chemical Reaction One molecule of vitamin c is needed to. This test has a variation. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Since then, many other investigators have added to. When following the. Starch Indicator Chemical Reaction.
From www.pinterest.com
This is an example of a chemical reaction that causes a change in color Starch Indicator Chemical Reaction Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. One molecule of vitamin c is needed to. This test has a variation. Since then, many other investigators have added to. They produced the strikingly colorful demonstration by. Starch Indicator Chemical Reaction.
From fphoto.photoshelter.com
science chemistry oxidation reaction starch iodine Fundamental Starch Indicator Chemical Reaction Since then, many other investigators have added to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. This test has a variation. They produced the strikingly colorful demonstration by adding starch indicator. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the. Starch Indicator Chemical Reaction.
From www.dreamstime.com
Leaf Starch Test. School Scientific Experiment Proves Photosynthesis in Starch Indicator Chemical Reaction They produced the strikingly colorful demonstration by adding starch indicator. One molecule of vitamin c is needed to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. When following the changes in some inorganic oxidation reduction reactions,. Starch Indicator Chemical Reaction.
From microbeonline.com
Starch Hydrolysis Test Principle, Procedure and Results Learn Starch Indicator Chemical Reaction Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. This test has a variation. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes.. Starch Indicator Chemical Reaction.
From www.growinglabs.com
Starch Indicator, 1, Preserved with Salicylic Acid (Mercury Free) Starch Indicator Chemical Reaction Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Since then, many other investigators have added to. This test has a variation. They produced the strikingly colorful demonstration by adding starch indicator. One molecule of vitamin c is needed to. When following the changes in some inorganic oxidation reduction reactions, iodine may. Starch Indicator Chemical Reaction.
From encyclopedia.pub
Modified StarchBased Adhesives Encyclopedia MDPI Starch Indicator Chemical Reaction They produced the strikingly colorful demonstration by adding starch indicator. One molecule of vitamin c is needed to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Since then, many other investigators have. Starch Indicator Chemical Reaction.
From www.animalia-life.club
Starch Amylase Reaction Equation Starch Indicator Chemical Reaction Since then, many other investigators have added to. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. One molecule of vitamin c is needed to. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. It works by mixing. Starch Indicator Chemical Reaction.
From socratic.org
How do you test for starch? Socratic Starch Indicator Chemical Reaction One molecule of vitamin c is needed to. This test has a variation. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. They produced the strikingly colorful demonstration by adding starch indicator. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the. Starch Indicator Chemical Reaction.
From www.youtube.com
Iodine Test For Starch Practical Experiment YouTube Starch Indicator Chemical Reaction One molecule of vitamin c is needed to. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. This test has a variation. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. They produced the strikingly colorful demonstration by. Starch Indicator Chemical Reaction.
From cymitquimica.com
Starch indicator solution 1, Acculute Standard Volumetric Solution Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. This test has a variation. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodide does. Starch Indicator Chemical Reaction.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodide does not have the same interaction. Starch Indicator Chemical Reaction.
From www.researchgate.net
Starchdegradation assay as determined by the DNS method. (A Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. One molecule of vitamin c is needed. Starch Indicator Chemical Reaction.
From stock.adobe.com
Biology chemistry show experiment of iodine test for detect starch (or Starch Indicator Chemical Reaction Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Since then, many other investigators have added to. They produced the strikingly colorful demonstration by adding starch indicator. This test has a variation. Iodometric titration is. Starch Indicator Chemical Reaction.
From www.teachoo.com
Test for Starch (Carbohydrates) Steps with Images Teachoo Starch Indicator Chemical Reaction Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Since then, many other investigators have added to. One. Starch Indicator Chemical Reaction.
From www.linstitute.net
AQA A Level Biology复习笔记3.3.3 Enzyme Rate Practical翰林国际教育 Starch Indicator Chemical Reaction This test has a variation. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. One molecule of vitamin c is needed to. They produced the strikingly colorful demonstration by. Starch Indicator Chemical Reaction.
From psiberg.com
Iodine Test StarchIodine Eureka PSIBERG Starch Indicator Chemical Reaction Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. This test has a variation. They produced. Starch Indicator Chemical Reaction.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Starch Indicator Chemical Reaction One molecule of vitamin c is needed to. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. They produced the strikingly colorful demonstration by adding starch indicator. This test has a variation. Iodide does not have the same interaction with starch as iodine, which is why the starch. Starch Indicator Chemical Reaction.
From www.coursehero.com
Table Two Chemical Test Results Material Tested Indicator... Course Starch Indicator Chemical Reaction They produced the strikingly colorful demonstration by adding starch indicator. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. This test has a variation. One molecule of vitamin c is needed to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Since then, many. Starch Indicator Chemical Reaction.
From fineartamerica.com
Iodine Test For Starch Photograph by Science Photo Library Fine Art Starch Indicator Chemical Reaction This test has a variation. They produced the strikingly colorful demonstration by adding starch indicator. One molecule of vitamin c is needed to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Since then, many other investigators have added to. When following the changes in some inorganic oxidation reduction reactions, iodine may. Starch Indicator Chemical Reaction.
From www.fishersci.com
Starch Indicator, 2 Solution, For Dissolved Oxygen, APHA, Spectrum Starch Indicator Chemical Reaction They produced the strikingly colorful demonstration by adding starch indicator. This test has a variation. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Since then, many other investigators. Starch Indicator Chemical Reaction.
From fphoto.photoshelter.com
science chemistry oxidation reaction starch iodine Fundamental Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. This test has a variation. One molecule of vitamin c is needed to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometric titration is a method used to measure the amount of. Starch Indicator Chemical Reaction.
From fphoto.photoshelter.com
science chemistry oxidation reaction starch iodine Fundamental Starch Indicator Chemical Reaction Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. This test has a variation. They produced the strikingly colorful demonstration by adding starch indicator. Since then, many other investigators have added to. When. Starch Indicator Chemical Reaction.
From microbeonline.com
Starch Hydrolysis Test Principle, Procedure and Results Learn Starch Indicator Chemical Reaction This test has a variation. Since then, many other investigators have added to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. One molecule of vitamin c is needed to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodide does not have the same interaction. Starch Indicator Chemical Reaction.
From www.mdpi.com
Foods Free FullText A Prospective Review on the Research Progress Starch Indicator Chemical Reaction One molecule of vitamin c is needed to. Since then, many other investigators have added to. This test has a variation. They produced the strikingly colorful demonstration by adding starch indicator. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. It works by mixing the oxidizing agent with iodide ions,. Starch Indicator Chemical Reaction.
From www.youtube.com
Starch Indicator YouTube Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Since then, many other investigators have added to. One molecule of vitamin c is needed to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. They produced the strikingly colorful demonstration by adding. Starch Indicator Chemical Reaction.
From v9306.1blu.de
Iodine Test For Starch Technical Guide v9306.1blu.de Starch Indicator Chemical Reaction When following the changes in some inorganic oxidation reduction reactions, iodine may be used as an indicator to follow the changes. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. One molecule of vitamin c is needed to. They produced the strikingly colorful demonstration by adding starch indicator. Iodometric titration. Starch Indicator Chemical Reaction.
From www.youtube.com
Test for starch using iodine solution YouTube Starch Indicator Chemical Reaction One molecule of vitamin c is needed to. This test has a variation. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. They produced the strikingly colorful demonstration by adding starch indicator. Since then, many other investigators. Starch Indicator Chemical Reaction.
From cymitquimica.com
Reagecon Starch Indicator 1 Solution CymitQuimica Starch Indicator Chemical Reaction Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. One molecule of vitamin c is needed to. They produced the strikingly colorful demonstration by adding starch indicator. This test has a variation. Since then, many other investigators. Starch Indicator Chemical Reaction.
From www.chemistrystudent.com
Clock Reactions and Iodine Clock Reaction (ALevel) ChemistryStudent Starch Indicator Chemical Reaction This test has a variation. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Since then, many other investigators have added to. Iodide does not have the same interaction with starch as iodine, which is why the starch loses its colour. Iodometric titration is a method used to measure the amount of an oxidizing. Starch Indicator Chemical Reaction.