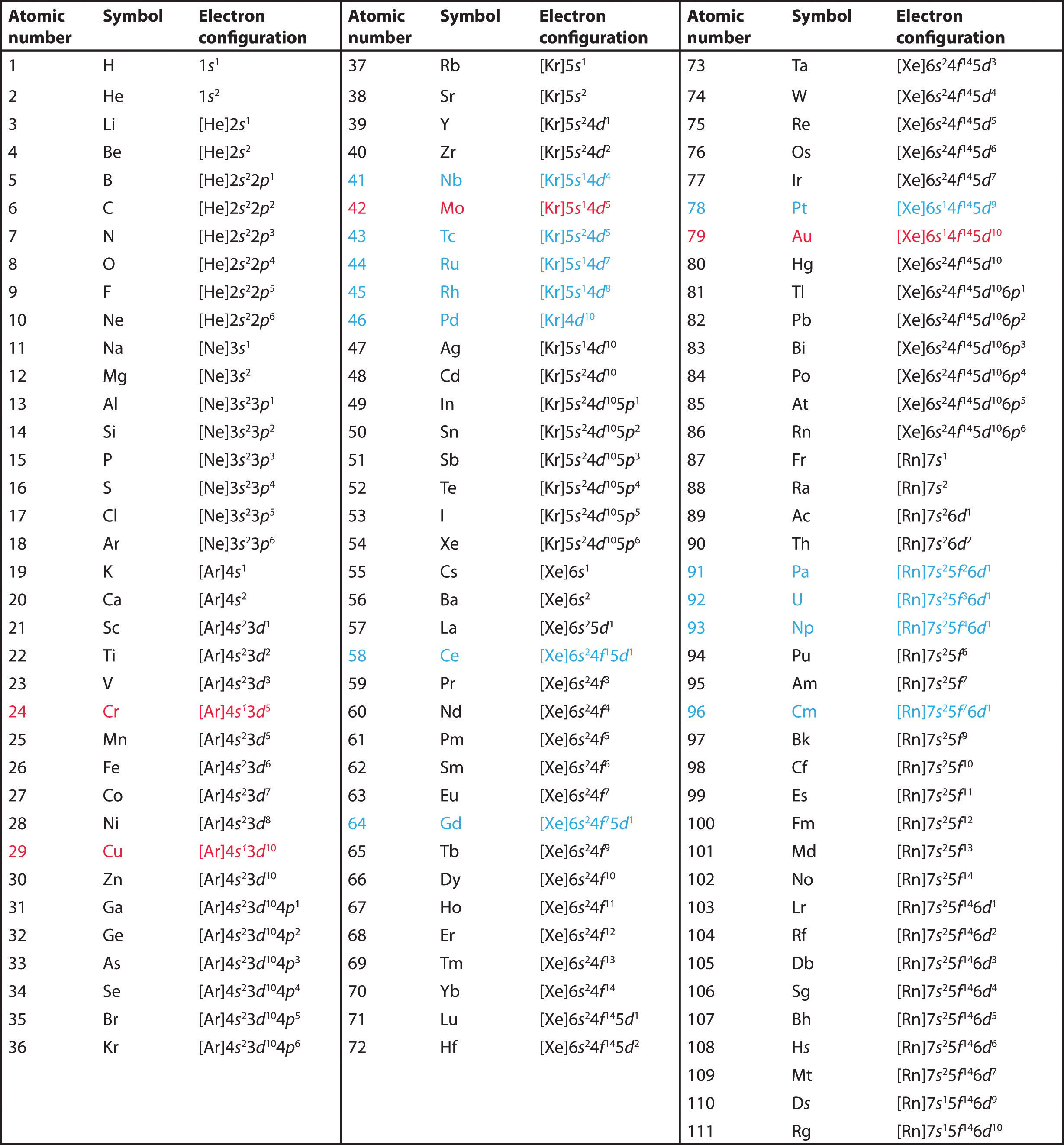
Electron Configuration For Tin 2+ . The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. This electron configuration shows that the tin ion (sn 4+) has four shells and. Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10.
from chem.libretexts.org
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. This electron configuration shows that the tin ion (sn 4+) has four shells and. Electron configuration chart of all elements is mentioned in the table below. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The shorthand electron configuration (or noble gas configuration) as well as.
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts
Electron Configuration For Tin 2+ This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Electron Configuration For Tin 2+ The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron configuration calculator. Electron Configuration For Tin 2+.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Electron Configuration For Tin 2+ The shorthand electron configuration (or noble gas configuration) as well as. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. The electron configuration of tin ion (sn 4+). Electron Configuration For Tin 2+.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Electron Configuration For Tin 2+ This electron configuration shows that the tin ion (sn 4+) has four shells and. The shorthand electron configuration (or noble gas configuration) as well as. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The tin electron configuration, represented as. Electron Configuration For Tin 2+.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Electron Configuration For Tin 2+ The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element.. Electron Configuration For Tin 2+.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Electron Configuration For Tin 2+ This electron configuration shows that the tin ion (sn 4+) has four shells and. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s. Electron Configuration For Tin 2+.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration For Tin 2+ The shorthand electron configuration (or noble gas configuration) as well as. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. To write the electron configuration of an atom,. Electron Configuration For Tin 2+.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron. Electron Configuration For Tin 2+.
From es.lambdageeks.com
Configuración de electrones de estaño (explicada para principiantes) Electron Configuration For Tin 2+ The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. This electron configuration shows that the tin ion (sn 4+) has four shells. Electron Configuration For Tin 2+.
From chem.libretexts.org
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. Electron configuration chart of all elements is mentioned in the table below. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron. Electron Configuration For Tin 2+.
From www.numerade.com
SOLVED(a) What is the electron configuration for an atom of tin? (b Electron Configuration For Tin 2+ The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s. Electron Configuration For Tin 2+.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Electron Configuration For Tin 2+ The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron configuration shows that the tin ion (sn 4+) has four shells and. To write the electron configuration of an atom, identify the energy level of interest and write the number of. Electron Configuration For Tin 2+.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron configuration shows that the tin ion (sn 4+) has four shells and. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements. Electron Configuration For Tin 2+.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Electron Configuration For Tin 2+ This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Electron Configuration For Tin 2+.
From holooly.com
Use the periodic table to write the shorthand electron configuration of Electron Configuration For Tin 2+ This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. This electron configuration shows that the tin ion (sn 4+) has four shells and. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Electron Configuration For Tin 2+.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The tin. Electron Configuration For Tin 2+.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Electron Configuration For Tin 2+ This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Electron Configuration For Tin 2+.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all. Electron Configuration For Tin 2+.
From hxecktzfj.blob.core.windows.net
Electron Configuration Chart Of Tin at Allison Napolitano blog Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron configuration shows that the tin ion (sn 4+) has four shells and.. Electron Configuration For Tin 2+.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration For Tin 2+ The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The tin electron. Electron Configuration For Tin 2+.
From sharihamish.blogspot.com
orbital diagram of tin ShariHamish Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron configuration shows that the tin ion (sn 4+) has four shells and. Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will. Electron Configuration For Tin 2+.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Electron Configuration For Tin 2+ This electron configuration shows that the tin ion (sn 4+) has four shells and. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration calculator will instantly. Electron Configuration For Tin 2+.
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Electron Configuration For Tin 2+.
From www.shutterstock.com
Tin Electron Configuration Propertiesvector Illustration Stock Vector Electron Configuration For Tin 2+ This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. This electron configuration shows that the tin ion (sn 4+) has four shells and. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s. Electron Configuration For Tin 2+.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration For Tin 2+ This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s. Electron Configuration For Tin 2+.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Electron Configuration For Tin 2+ This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10.. Electron Configuration For Tin 2+.
From www.webelements.com
Elements Periodic Table » Tin » properties of free atoms Electron Configuration For Tin 2+ The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s. Electron Configuration For Tin 2+.
From ar.inspiredpencil.com
Tin Atomic Structure Electron Configuration For Tin 2+ The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin is 1s2 2s2. Electron Configuration For Tin 2+.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Electron Configuration For Tin 2+.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Electron Configuration For Tin 2+ This electron configuration shows that the tin ion (sn 4+) has four shells and. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of. Electron Configuration For Tin 2+.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. The electron configuration. Electron Configuration For Tin 2+.
From www.colourbox.com
Symbol and electron diagram for Tin illustration Stock Vector Colourbox Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration chart of all elements is mentioned in the table below.. Electron Configuration For Tin 2+.
From www.youtube.com
Sn^2+,Sn^4+ and Sn (Tin and Tin Ions) Electron ConfigurationCrash Electron Configuration For Tin 2+ The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2. This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin ion (sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. To write. Electron Configuration For Tin 2+.
From www.chegg.com
Solved 5 What is the electron configuration for tin (Sn)? (4 Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of tin ion (sn 4+). Electron Configuration For Tin 2+.
From www.chemistry-online.com
Tin Chemistry Online Electron Configuration For Tin 2+ To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: This electron configuration shows that the tin ion (sn 4+) has four shells and. The electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2.. Electron Configuration For Tin 2+.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Electron Configuration For Tin 2+ The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons. This electron configuration shows that the tin ion (sn 4+) has four shells and. This electron configuration calculator will. Electron Configuration For Tin 2+.