Which Best Explains The Surface Tension Of Water Quizlet . Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Fundamentals of momentum, heat and mass transfer. Read slides 17 and 18 on surface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of a penny because of this property. Some insects can stride across the water due to the combination of their legs and the high surface. Water has high surface tension; Meaning it is adhesive and elastic. It tends to aggregate in drops rather. Water droplets take a spherical shape (as pictured) because of. **cohesive forces:** water molecules are attracted to each other,. Water has a high surface tension. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Here are a few key points to explain surface tension of water:
from www.chegg.com
Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Meaning it is adhesive and elastic. Here are a few key points to explain surface tension of water: Water has a high surface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. **cohesive forces:** water molecules are attracted to each other,. Read slides 17 and 18 on surface tension. Water beads up on the surface of a penny because of this property. Water droplets take a spherical shape (as pictured) because of. Fundamentals of momentum, heat and mass transfer.
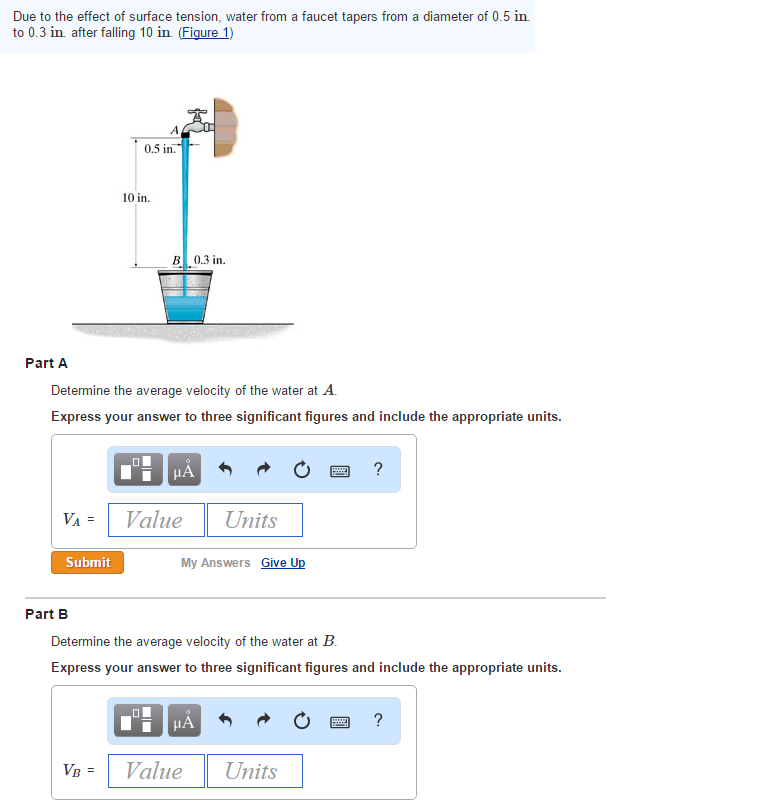
Solved Due to the effect of surface tension, water from a
Which Best Explains The Surface Tension Of Water Quizlet Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Water has high surface tension; Read slides 17 and 18 on surface tension. **cohesive forces:** water molecules are attracted to each other,. Here are a few key points to explain surface tension of water: Water has a high surface tension. Water droplets take a spherical shape (as pictured) because of. It tends to aggregate in drops rather. Water beads up on the surface of a penny because of this property. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Some insects can stride across the water due to the combination of their legs and the high surface. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Fundamentals of momentum, heat and mass transfer. Meaning it is adhesive and elastic.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. Water beads up on the surface of a penny because of this property. Fundamentals of momentum, heat and mass transfer. Some insects can stride across the water due to the combination of their legs and the high surface. Water droplets take a spherical shape (as pictured) because of. Water has high surface tension; **cohesive. Which Best Explains The Surface Tension Of Water Quizlet.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Which Best Explains The Surface Tension Of Water Quizlet Some insects can stride across the water due to the combination of their legs and the high surface. Water has high surface tension; Meaning it is adhesive and elastic. It tends to aggregate in drops rather. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Water has a high surface tension. Water beads up on. Which Best Explains The Surface Tension Of Water Quizlet.
From www.chegg.com
Solved 7 Explain surface tension and give a real world Which Best Explains The Surface Tension Of Water Quizlet Here are a few key points to explain surface tension of water: Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Read slides 17 and 18 on surface tension. Water has high surface tension; Fundamentals of momentum, heat and mass transfer. **cohesive forces:** water molecules are attracted to each other,. Study with quizlet and memorize. Which Best Explains The Surface Tension Of Water Quizlet.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Which Best Explains The Surface Tension Of Water Quizlet Water droplets take a spherical shape (as pictured) because of. Meaning it is adhesive and elastic. It tends to aggregate in drops rather. **cohesive forces:** water molecules are attracted to each other,. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of a penny because of this. Which Best Explains The Surface Tension Of Water Quizlet.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. Water beads up on the surface of a penny because of this property. Water droplets take a spherical shape (as pictured) because of. Meaning it is adhesive and elastic. It tends to aggregate in drops rather. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Some insects can stride. Which Best Explains The Surface Tension Of Water Quizlet.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Which Best Explains The Surface Tension Of Water Quizlet Water beads up on the surface of a penny because of this property. Meaning it is adhesive and elastic. Here are a few key points to explain surface tension of water: Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Water has high surface tension; Study with quizlet. Which Best Explains The Surface Tension Of Water Quizlet.
From www.greenkidcrafts.com
Water Surface Tension Experiment for Kids Green Kid Crafts Which Best Explains The Surface Tension Of Water Quizlet Read slides 17 and 18 on surface tension. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Some insects can stride across the water due to the combination of their legs and the high surface. Here are a few key points to explain surface tension of water: Water has high surface tension; Water has a. Which Best Explains The Surface Tension Of Water Quizlet.
From mirjamglessmer.com
Trying to understand surface tension Adventures in Oceanography and Which Best Explains The Surface Tension Of Water Quizlet Fundamentals of momentum, heat and mass transfer. Read slides 17 and 18 on surface tension. Here are a few key points to explain surface tension of water: Water has a high surface tension. **cohesive forces:** water molecules are attracted to each other,. Water has high surface tension; Water beads up on the surface of a penny because of this property.. Which Best Explains The Surface Tension Of Water Quizlet.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Which Best Explains The Surface Tension Of Water Quizlet Some insects can stride across the water due to the combination of their legs and the high surface. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. It tends to aggregate in drops rather. **cohesive forces:** water molecules are attracted to each other,. Meaning it is adhesive and elastic. Study with quizlet and. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
To study surface tension of water by capillary rise method. YouTube Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. **cohesive forces:** water molecules are attracted to each other,. It tends to aggregate in drops rather. Water beads up on the surface of a penny because of this property. Fundamentals of momentum, heat and mass transfer. Read slides 17 and. Which Best Explains The Surface Tension Of Water Quizlet.
From byjus.com
Explain the surface tension phenomenon with examples. Which Best Explains The Surface Tension Of Water Quizlet Fundamentals of momentum, heat and mass transfer. Read slides 17 and 18 on surface tension. Water has high surface tension; **cohesive forces:** water molecules are attracted to each other,. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Some insects can stride across the water due to the. Which Best Explains The Surface Tension Of Water Quizlet.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Which Best Explains The Surface Tension Of Water Quizlet Water has high surface tension; Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Here are a few key points to explain surface tension of water: Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Read slides 17 and 18 on surface tension. Water has a high surface. Which Best Explains The Surface Tension Of Water Quizlet.
From fyoqevkxu.blob.core.windows.net
Surface Tension Quizlet at Lawrence Gardner blog Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. **cohesive forces:** water molecules are attracted to each other,. Water has high surface tension; Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of a penny because of this property. Water droplets take a spherical shape (as pictured) because of. Study. Which Best Explains The Surface Tension Of Water Quizlet.
From kidpillar.com
What is Surface Tension + Fun Experiments on Surface Tension Which Best Explains The Surface Tension Of Water Quizlet Meaning it is adhesive and elastic. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Here are a few key points to explain surface tension of water: Water has a high surface tension. Water has high surface tension; **cohesive forces:** water molecules are attracted to each other,. Water. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
Surface Tension of given Liquid (Water) by Jaeger's Method YouTube Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. It tends to aggregate in drops rather. Read slides 17 and 18 on surface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of a penny because of this property. Some insects can stride across the water due to the. Which Best Explains The Surface Tension Of Water Quizlet.
From anabelximorales.blogspot.com
Which Best Explains the Surface Tension of Water Capillary Action Which Best Explains The Surface Tension Of Water Quizlet Fundamentals of momentum, heat and mass transfer. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Meaning it is adhesive and elastic. **cohesive forces:** water molecules are attracted to each other,. Some insects can stride across the water due to the combination of their legs and the high surface. Water has a high surface tension.. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
Lecture 3 Liquid state Applications of surface tension in daily life Which Best Explains The Surface Tension Of Water Quizlet Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Water has a high surface tension. Meaning it is adhesive and elastic. **cohesive forces:** water molecules are attracted to each other,. Water has high surface tension; Here are a few key points to explain surface tension of water: It. Which Best Explains The Surface Tension Of Water Quizlet.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Which Best Explains The Surface Tension Of Water Quizlet Meaning it is adhesive and elastic. Here are a few key points to explain surface tension of water: It tends to aggregate in drops rather. Water droplets take a spherical shape (as pictured) because of. Some insects can stride across the water due to the combination of their legs and the high surface. Water has a high surface tension. Read. Which Best Explains The Surface Tension Of Water Quizlet.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Which Best Explains The Surface Tension Of Water Quizlet **cohesive forces:** water molecules are attracted to each other,. Meaning it is adhesive and elastic. Water beads up on the surface of a penny because of this property. It tends to aggregate in drops rather. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Study with quizlet and memorize flashcards containing terms like which best. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
Surface Tension of water 🚤 animated video YouTube Which Best Explains The Surface Tension Of Water Quizlet Water beads up on the surface of a penny because of this property. Water has a high surface tension. Read slides 17 and 18 on surface tension. Here are a few key points to explain surface tension of water: Fundamentals of momentum, heat and mass transfer. Study with quizlet and memorize flashcards containing terms like which best explains why an. Which Best Explains The Surface Tension Of Water Quizlet.
From mavink.com
Water Surface Tension Chart Which Best Explains The Surface Tension Of Water Quizlet Fundamentals of momentum, heat and mass transfer. Water beads up on the surface of a penny because of this property. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Read slides 17 and 18. Which Best Explains The Surface Tension Of Water Quizlet.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Which Best Explains The Surface Tension Of Water Quizlet Here are a few key points to explain surface tension of water: Meaning it is adhesive and elastic. Some insects can stride across the water due to the combination of their legs and the high surface. **cohesive forces:** water molecules are attracted to each other,. Read slides 17 and 18 on surface tension. Water has a high surface tension. Study. Which Best Explains The Surface Tension Of Water Quizlet.
From www.biolinscientific.com
Surface tension of water Why is it so high? Which Best Explains The Surface Tension Of Water Quizlet Here are a few key points to explain surface tension of water: **cohesive forces:** water molecules are attracted to each other,. Some insects can stride across the water due to the combination of their legs and the high surface. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Meaning it is adhesive and elastic. Water. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
Water's surface tension physics experiment YouTube Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Read slides 17 and 18 on surface tension. It tends to aggregate in drops rather. Water beads up on the surface of a penny because of this property. Here are a few key. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
Walking on Water!! Surface Tension YouTube Which Best Explains The Surface Tension Of Water Quizlet Fundamentals of momentum, heat and mass transfer. It tends to aggregate in drops rather. Read slides 17 and 18 on surface tension. Water droplets take a spherical shape (as pictured) because of. Meaning it is adhesive and elastic. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Study with quizlet and memorize flashcards. Which Best Explains The Surface Tension Of Water Quizlet.
From www.youtube.com
Surface Tension of Water Explained YouTube Which Best Explains The Surface Tension Of Water Quizlet Fundamentals of momentum, heat and mass transfer. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Water has high surface tension; Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. It tends to aggregate in drops rather. Read slides 17 and 18 on. Which Best Explains The Surface Tension Of Water Quizlet.
From guidemanualspyglasses.z14.web.core.windows.net
How To Explain Surface Tension Which Best Explains The Surface Tension Of Water Quizlet Water beads up on the surface of a penny because of this property. It tends to aggregate in drops rather. Fundamentals of momentum, heat and mass transfer. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Water has a high surface tension. Water has high surface tension; Water droplets take a spherical shape (as pictured). Which Best Explains The Surface Tension Of Water Quizlet.
From fyoqevkxu.blob.core.windows.net
Surface Tension Quizlet at Lawrence Gardner blog Which Best Explains The Surface Tension Of Water Quizlet Meaning it is adhesive and elastic. Here are a few key points to explain surface tension of water: **cohesive forces:** water molecules are attracted to each other,. Water has a high surface tension. Water beads up on the surface of a penny because of this property. Read slides 17 and 18 on surface tension. Study with quizlet and memorize flashcards. Which Best Explains The Surface Tension Of Water Quizlet.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action Which Best Explains The Surface Tension Of Water Quizlet Here are a few key points to explain surface tension of water: Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Meaning it is adhesive and elastic. Water has high surface tension; Fundamentals of momentum, heat and mass transfer. Read slides 17 and 18 on surface tension. Water beads up on the surface of a. Which Best Explains The Surface Tension Of Water Quizlet.
From smartquizbasket.blogspot.com
Smart Quiz Basket Surface Tension Definition Chemistry Which Best Explains The Surface Tension Of Water Quizlet **cohesive forces:** water molecules are attracted to each other,. It tends to aggregate in drops rather. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Fundamentals of momentum, heat and mass transfer. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high.. Which Best Explains The Surface Tension Of Water Quizlet.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Which Best Explains The Surface Tension Of Water Quizlet Meaning it is adhesive and elastic. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Water has high surface tension; Read slides 17 and 18 on surface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water droplets take a. Which Best Explains The Surface Tension Of Water Quizlet.
From byjus.com
Explain the surface tension phenomenon with examples. Which Best Explains The Surface Tension Of Water Quizlet Water has high surface tension; Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Some insects can stride across the water due to the combination of their legs and the high surface.. Which Best Explains The Surface Tension Of Water Quizlet.
From www.chegg.com
Solved Due to the effect of surface tension, water from a Which Best Explains The Surface Tension Of Water Quizlet It tends to aggregate in drops rather. **cohesive forces:** water molecules are attracted to each other,. Some insects can stride across the water due to the combination of their legs and the high surface. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Water has high surface tension;. Which Best Explains The Surface Tension Of Water Quizlet.
From mungfali.com
Water Surface Tension Bubble Which Best Explains The Surface Tension Of Water Quizlet Some insects can stride across the water due to the combination of their legs and the high surface. Water has a high surface tension. It tends to aggregate in drops rather. Study with quizlet and memorize flashcards containing terms like which best explains why an iceberg floats?, which best explains the high. Surface tension is a phenomenon in which the. Which Best Explains The Surface Tension Of Water Quizlet.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… Which Best Explains The Surface Tension Of Water Quizlet Water has a high surface tension. It tends to aggregate in drops rather. Water has high surface tension; Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Study with quizlet and memorize flashcards containing terms like hydrogen bonding increases ____ in. Water beads up on the surface of a penny because of this. Which Best Explains The Surface Tension Of Water Quizlet.