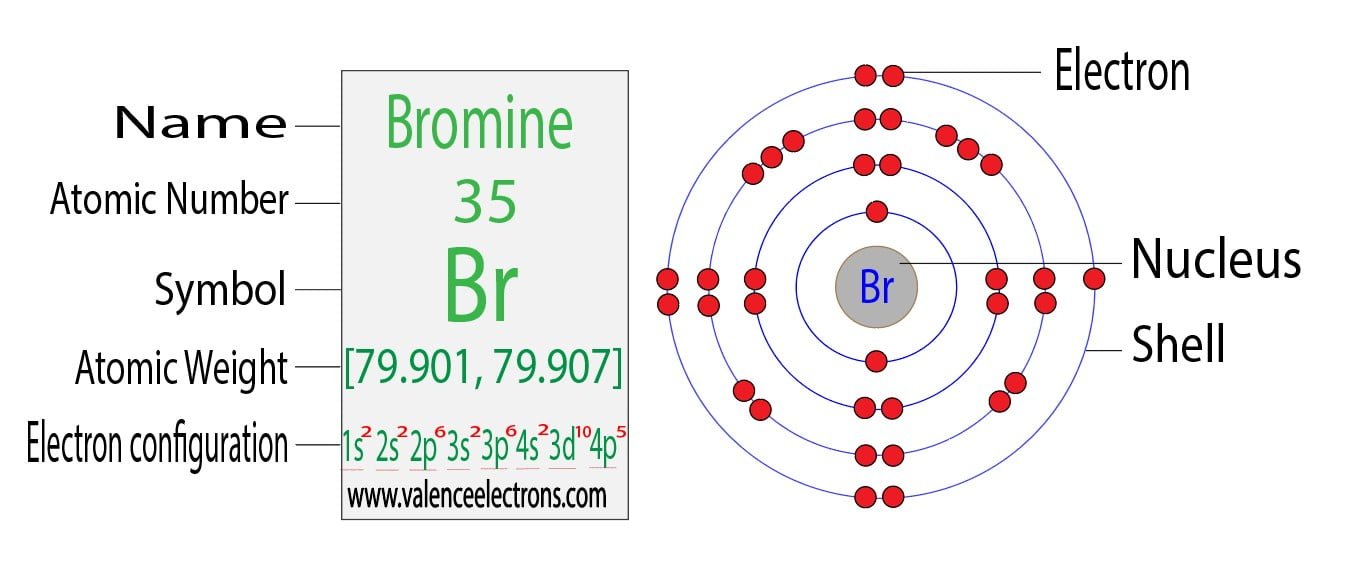
Bromine Atom Have Valence Electrons . Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Valence electrons can be found in the p and s highest energy orbitals. Bromine has an electron configuration of #1s^2 2s^2 2p^6. Iron is a transition metal, so the number. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. But for most of the transition. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5.
from www.animalia-life.club
Valence electrons found in the s and p orbitals of the highest energy. Valence electrons can be found in the p and s highest energy orbitals. Bromine has seven valence electrons. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. But for most of the transition. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a transition metal, so the number. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Bromine has an electron configuration of #1s^2 2s^2 2p^6.
Electron Configuration For Bromine
Bromine Atom Have Valence Electrons The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Bromine has an electron configuration of #1s^2 2s^2 2p^6. But for most of the transition. Valence electrons can be found in the p and s highest energy orbitals. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Iron is a transition metal, so the number. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Valence electrons found in the s and p orbitals of the highest energy. Bromine has seven valence electrons.
From exooeohnk.blob.core.windows.net
Bromine Total Number Of Valence Electrons at Rebeca Henshaw blog Bromine Atom Have Valence Electrons Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons. Bromine Atom Have Valence Electrons.
From joiglahlj.blob.core.windows.net
Electron Configuration Of Bromine at Johanna Brewton blog Bromine Atom Have Valence Electrons But for most of the transition. Iron is a transition metal, so the number. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has an electron configuration of #1s^2 2s^2 2p^6. The total number of electrons present in the valence shell. Bromine Atom Have Valence Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Atom Have Valence Electrons But for most of the transition. Bromine has seven valence electrons. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the. Bromine Atom Have Valence Electrons.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Atom Have Valence Electrons Bromine has an electron configuration of #1s^2 2s^2 2p^6. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a transition metal, so the number. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in. Bromine Atom Have Valence Electrons.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Bromine Atom Have Valence Electrons Bromine has seven valence electrons. Bromine has an electron configuration of #1s^2 2s^2 2p^6. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons But for most of the transition. Valence electrons can be found in the p and s highest energy orbitals. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Valence electrons found in. Bromine Atom Have Valence Electrons.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Atom Have Valence Electrons The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. But for most of the transition. The. Bromine Atom Have Valence Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Atom Have Valence Electrons But for most of the transition. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Valence electrons found in the s and p orbitals of the highest energy. Br has an electron configuration of1s 2 2s 2. Bromine Atom Have Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Atom Have Valence Electrons Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. But for most of the transition. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p. Bromine Atom Have Valence Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Atom Have Valence Electrons Valence electrons found in the s and p orbitals of the highest energy. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a transition metal, so the number. Br has an electron configuration of1s 2 2s 2 2p 6 3s. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons Valence electrons can be found in the p and s highest energy orbitals. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has an electron configuration of #1s^2 2s^2 2p^6. Br has an electron configuration of1s 2 2s 2 2p 6. Bromine Atom Have Valence Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Have Valence Electrons Iron is a transition metal, so the number. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Bromine has an electron configuration of #1s^2 2s^2 2p^6. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. But for most of the. Bromine Atom Have Valence Electrons.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Atom Have Valence Electrons Valence electrons can be found in the p and s highest energy orbitals. Iron is a transition metal, so the number. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Bromine has. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Valence electrons found in the s and p orbitals of the highest energy. Iron is a transition metal, so the number. Bromine has seven valence electrons. The valence electrons are there in the 4s and 4p orbitals which gives bromine. Bromine Atom Have Valence Electrons.
From exooeohnk.blob.core.windows.net
Bromine Total Number Of Valence Electrons at Rebeca Henshaw blog Bromine Atom Have Valence Electrons Iron is a transition metal, so the number. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Br has an electron configuration. Bromine Atom Have Valence Electrons.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? Bromine Atom Have Valence Electrons Bromine has seven valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Iron is a transition metal, so the number. But for most of the transition. Valence electrons can be found in the p and s highest energy orbitals. The total number of electrons present in the. Bromine Atom Have Valence Electrons.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Atom Have Valence Electrons Valence electrons can be found in the p and s highest energy orbitals. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present in. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Bromine has an electron configuration of #1s^2 2s^2 2p^6. Valence electrons can be found in the p and s highest energy orbitals. Iron. Bromine Atom Have Valence Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Atom Have Valence Electrons Iron is a transition metal, so the number. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Bromine has seven valence electrons. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Valence electrons found in the s and p orbitals of. Bromine Atom Have Valence Electrons.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Bromine Atom Have Valence Electrons The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of. Bromine Atom Have Valence Electrons.
From www.dreamstime.com
Bromine stock illustration. Illustration of render, formula 139651101 Bromine Atom Have Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Valence electrons can be found in the p and s highest energy. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. The valence electrons are there in the 4s and 4p orbitals which gives. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. But for most of the transition. Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a. Bromine Atom Have Valence Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Have Valence Electrons Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of. Bromine Atom Have Valence Electrons.
From www.slideserve.com
PPT Atoms & Chemical Bonding PowerPoint Presentation, free download Bromine Atom Have Valence Electrons Valence electrons can be found in the p and s highest energy orbitals. But for most of the transition. Iron is a transition metal, so the number. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2. Bromine Atom Have Valence Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Atom Have Valence Electrons Valence electrons found in the s and p orbitals of the highest energy. Valence electrons can be found in the p and s highest energy orbitals. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Iron is. Bromine Atom Have Valence Electrons.
From narodnatribuna.info
This Is Bromine Atomic Number 35 Atomic Mass 79904 Bromine Atom Have Valence Electrons Iron is a transition metal, so the number. Bromine has an electron configuration of #1s^2 2s^2 2p^6. But for most of the transition. Valence electrons found in the s and p orbitals of the highest energy. Valence electrons can be found in the p and s highest energy orbitals. Only the electrons in the outmost shell are valance electrons.all but. Bromine Atom Have Valence Electrons.
From loehniqha.blob.core.windows.net
What Is The Valency Of Bromide at Manuel Chappelle blog Bromine Atom Have Valence Electrons The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Valence electrons found in the s and p orbitals of the highest energy. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a. Bromine Atom Have Valence Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Atom Have Valence Electrons But for most of the transition. The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has an electron configuration of #1s^2 2s^2 2p^6. Br. Bromine Atom Have Valence Electrons.
From proper-cooking.info
Bromine Valence Electrons Bromine Atom Have Valence Electrons The valence electrons are there in the 4s and 4p orbitals which gives bromine 7 valence electrons. Bromine has an electron configuration of #1s^2 2s^2 2p^6. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Iron is. Bromine Atom Have Valence Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Atom Have Valence Electrons Iron is a transition metal, so the number. Valence electrons found in the s and p orbitals of the highest energy. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. The total number of electrons present in the valence shell of an. Bromine Atom Have Valence Electrons.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Atom Have Valence Electrons Iron is a transition metal, so the number. Bromine has seven valence electrons. Valence electrons found in the s and p orbitals of the highest energy. Valence electrons can be found in the p and s highest energy orbitals. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5.. Bromine Atom Have Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Have Valence Electrons But for most of the transition. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in. Bromine Atom Have Valence Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine Atom Have Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the. Bromine Atom Have Valence Electrons.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Bromine Atom Have Valence Electrons Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. But for most of the transition. Valence electrons found in the s and p orbitals of the highest energy. Iron is a transition metal, so the number. Valence. Bromine Atom Have Valence Electrons.