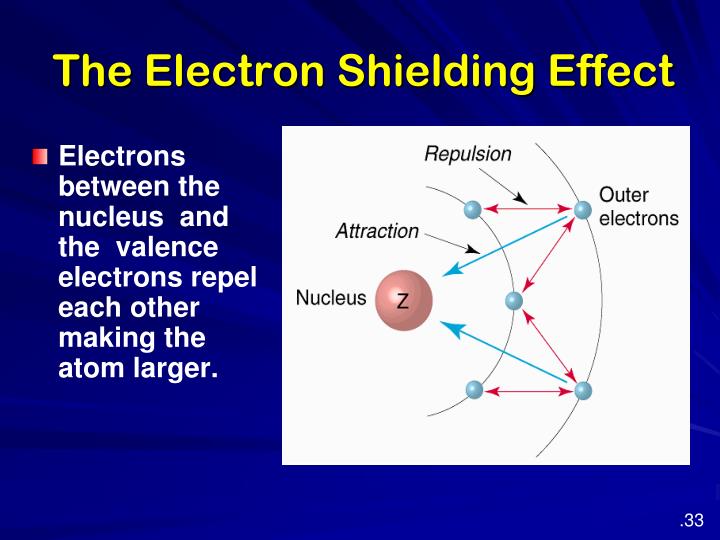
What Is Shielding Effect Of Electrons . Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. However, due to the repulsive forces from the inner electrons, the.
from www.slideserve.com
Hence, the nucleus has less grip on the outer electrons and are shielded from them. However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell.
PPT Periodic Table PowerPoint Presentation ID2834450
What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From scienceinfo.com
Shielding effect What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Hence, the nucleus has less grip on the outer electrons and are shielded from them. However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering. What Is Shielding Effect Of Electrons.
From ceuwfxbf.blob.core.windows.net
What Does A Shielding Electron at Pamela Mccarthy blog What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering. What Is Shielding Effect Of Electrons.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Shielding Effect Of Electrons Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From www.shutterstock.com
Proton electron charge Images, Stock Photos & Vectors Shutterstock What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation ID2834450 What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is Shielding Effect Of Electrons Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.chemistrysteps.com
NMR Chemical Shift ppm, Upfield, Downfield Chemistry Steps What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From byjus.com
What is effective nuclear charge and shielding effect? What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From ecurrencythailand.com
What Is The Electron Shielding Effect? Best 7 Answer What Is Shielding Effect Of Electrons Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the. What Is Shielding Effect Of Electrons.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Shielding Effect Of Electrons Hence, the nucleus has less grip on the outer electrons and are shielded from them. However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.
From dxoegqbbm.blob.core.windows.net
Electron Shielding Effect Ionization Energy at Elizabeth Shank blog What Is Shielding Effect Of Electrons Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. However, due to the repulsive forces from the inner electrons, the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. What Is Shielding Effect Of Electrons.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Electrons However, due to the repulsive forces from the inner electrons, the. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in the outermost shell. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. What Is Shielding Effect Of Electrons.