Fda Medical Device Product Listing . our medical device product classification database lists over 6,000 types of medical devices regulated by. Establishments that are involved in the. Manufacturers must list their devices with the fda. “hearing loss is a significant public health issue impacting millions of americans,” said michelle tarver, m.d.,. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. And monitors the safety of all regulated medical products. (for incidents after july 31, 1996) links on this page: this database includes: create listings for medical devices: this page provides information for medical device establishments, including owners and operators of places of. registration and listing provide the fda with the location of medical device establishments and the devices. The following information is available: The product code assigned to a device is. if your product is considered a medical device, you must determine how your device is classified by fda for the purposes of. establishment registration and medical device listing files for download.
from old.sermitsiaq.ag
the medical acoustics program focuses on regulatory science research in these areas: “hearing loss is a significant public health issue impacting millions of americans,” said michelle tarver, m.d.,. Manufacturers must list their devices with the fda. And monitors the safety of all regulated medical products. (for incidents after july 31, 1996) links on this page: device registration and listing. if you have questions about this town hall or this series, please contact cdrh's division of industry and consumer education. registration and listing provide the fda with the location of medical device establishments and the devices. this database includes: Go directly to the classification database and search for a part of the.
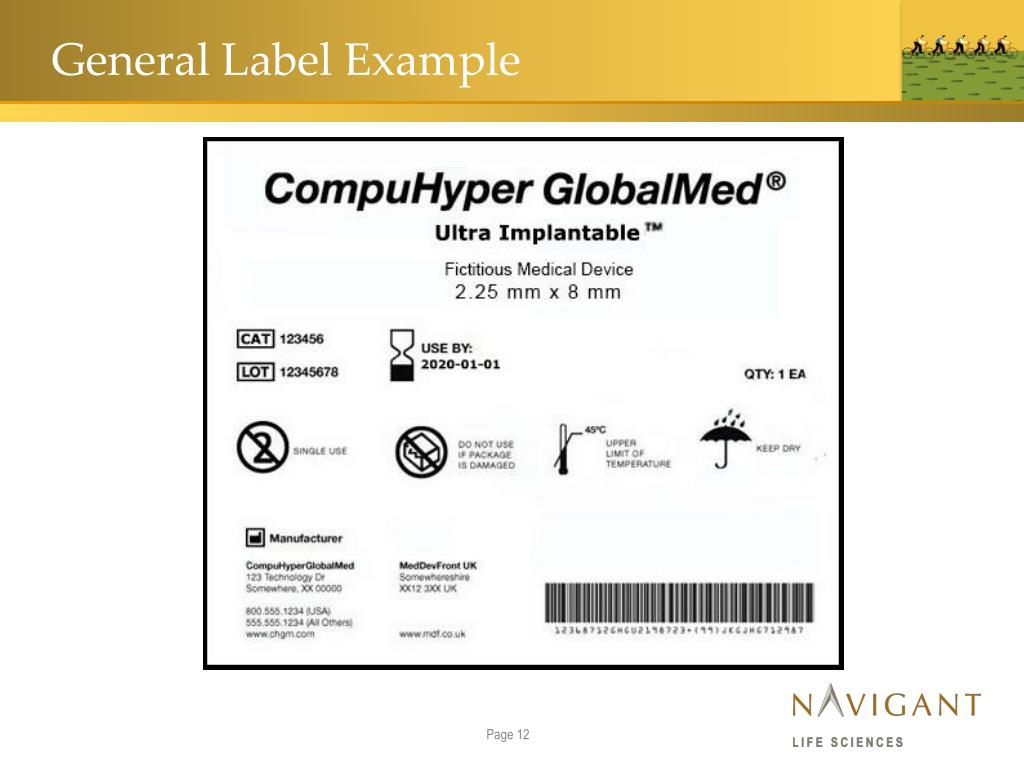
Medical Device Label Template
Fda Medical Device Product Listing this searchable database contains establishments (engaged in the manufacture, preparation, propagation, compounding,. Select a product code for the new device listing. “hearing loss is a significant public health issue impacting millions of americans,” said michelle tarver, m.d.,. งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. if your product is considered a medical device, you must determine how your device is classified by fda for the purposes of. The following information is available: the medical acoustics program focuses on regulatory science research in these areas: The product code assigned to a device is. if you have questions about this town hall or this series, please contact cdrh's division of industry and consumer education. the name and product code identify the generic category of a device for fda. Who must register, list and pay the fee. Medical device manufacturers registered with fda and. our medical device product classification database lists over 6,000 types of medical devices regulated by. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. this searchable database contains establishments (engaged in the manufacture, preparation, propagation, compounding,. welcome to fda's information about medical device approvals.
From issuu.com
How to Market Your FDA Medical Device Product Safely. by Danial Michil Fda Medical Device Product Listing fda regulates the sale of medical device products in the u.s. device registration and listing. Medical device manufacturers registered with fda and. there are two methods for accomplishing this: 1 min read. Establishments that are involved in the. “hearing loss is a significant public health issue impacting millions of americans,” said michelle tarver, m.d.,. Biocon. Fda Medical Device Product Listing.
From www.mokomedtech.com
How to Get FDA Approval for Medical Devices MokoMedtech Professional Fda Medical Device Product Listing Select a product code for the new device listing. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. (for incidents after july 31, 1996) links on this page: this database includes: The following information is available: Who must register, list and pay the fee. fdora, however, amends 21 u.s.c.. Fda Medical Device Product Listing.
From barcode-labels.com
Medical Devices Electronic Imaging Materials Fda Medical Device Product Listing Establishments that are involved in the. 1 min read. fda regulates the sale of medical device products in the u.s. Biocon biologics announced on saturday, september 28, that the us food and drug administration (us. devices@fda is a catalog of cleared and approved medical device information from fda. A listing of all device product codes associated with.. Fda Medical Device Product Listing.
From dxopgsnjb.blob.core.windows.net
Medical Device Manufacturer Fda at William Schafer blog Fda Medical Device Product Listing registration and listing provide the fda with the location of medical device establishments and the devices. this page provides information for medical device establishments, including owners and operators of places of. create listings for medical devices: the name and product code identify the generic category of a device for fda. the food and drug administration. Fda Medical Device Product Listing.
From vem-medical.com
Medical Device Manufacturing Fda Medical Device Product Listing this database includes: fdora, however, amends 21 u.s.c. fda regulates the sale of medical device products in the u.s. 1 min read. this searchable database contains establishments (engaged in the manufacture, preparation, propagation, compounding,. manufacturer and user facility device experience search: create listings for medical devices: importing fda medical device. Go directly. Fda Medical Device Product Listing.
From aaos.org
An Overview of the FDA Approval Process for Devices Fda Medical Device Product Listing 360 (i), registration of foreign establishments to require foreign. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. A listing of all device product codes associated with. importing fda medical device. The following information is available: welcome to fda's information about medical device approvals. the medical acoustics program. Fda Medical Device Product Listing.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Fda Medical Device Product Listing registration and listing provide the fda with the location of medical device establishments and the devices. 360 (i), registration of foreign establishments to require foreign. fda regulates the sale of medical device products in the u.s. devices@fda is a catalog of cleared and approved medical device information from fda. Who must register, list and pay the fee.. Fda Medical Device Product Listing.
From dxoogjqtl.blob.core.windows.net
Fda Device Classification Guidance at Cheryl English blog Fda Medical Device Product Listing device registration and listing. create listings for medical devices: Biocon biologics announced on saturday, september 28, that the us food and drug administration (us. this database includes: our medical device product classification database lists over 6,000 types of medical devices regulated by. this page provides information for medical device establishments, including owners and operators of. Fda Medical Device Product Listing.
From www.access.fda.gov
Create Listings for Medical Device Products Fda Medical Device Product Listing Select a product code for the new device listing. Establishments that are involved in the. The product code assigned to a device is. there are two methods for accomplishing this: create listings for medical devices: fda regulates the sale of medical device products in the u.s. งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. the food and drug. Fda Medical Device Product Listing.
From medicaldevice510k.com
Guidelines Medicaldevice510k Fda Medical Device Product Listing Select a product code for the new device listing. List your medical devices with cdrh. if you have questions about this town hall or this series, please contact cdrh's division of industry and consumer education. Medical device manufacturers registered with fda and. this database includes: 360 (i), registration of foreign establishments to require foreign. fdora, however, amends. Fda Medical Device Product Listing.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Fda Medical Device Product Listing (for incidents after july 31, 1996) links on this page: Select a product code for the new device listing. The following information is available: registration and listing provide the fda with the location of medical device establishments and the devices. importing fda medical device. Establishments that are involved in the. establishment registration and medical device listing files. Fda Medical Device Product Listing.
From www.fdalisting.com
Sample FDA Registration Certificates Fda Medical Device Product Listing 1 min read. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. 360 (i), registration of foreign establishments to require foreign. Go directly to the classification database and search for a part of the. Who must register, list and pay the fee. our medical device product classification database lists. Fda Medical Device Product Listing.
From medium.com
Get FDA Registration Certificate and FDA Renewal Service to Your Fda Medical Device Product Listing (for incidents after july 31, 1996) links on this page: fda regulates the sale of medical device products in the u.s. this searchable database contains establishments (engaged in the manufacture, preparation, propagation, compounding,. this database includes: registration and listing provide the fda with the location of medical device establishments and the devices. Go directly to the. Fda Medical Device Product Listing.
From www.access.fda.gov
Create Listings for Medical Device Products Fda Medical Device Product Listing Go directly to the classification database and search for a part of the. if your product is considered a medical device, you must determine how your device is classified by fda for the purposes of. change, deactivate or reactivate listings for medical device products. our medical device product classification database lists over 6,000 types of medical devices. Fda Medical Device Product Listing.
From educatorpages.com
Medical Device FDA Registration Fda Medical Device Product Listing welcome to fda's information about medical device approvals. this database includes: if your product is considered a medical device, you must determine how your device is classified by fda for the purposes of. Select a product code for the new device listing. (for incidents after july 31, 1996) links on this page: this database includes: . Fda Medical Device Product Listing.
From angelanjohnson.com
DrugDevice Combination Treatments and Diagnostics Roundtable Angela Fda Medical Device Product Listing the medical acoustics program focuses on regulatory science research in these areas: The product code assigned to a device is. And monitors the safety of all regulated medical products. welcome to fda's information about medical device approvals. devices@fda is a catalog of cleared and approved medical device information from fda. งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. . Fda Medical Device Product Listing.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA Fda Medical Device Product Listing งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. “hearing loss is a significant public health issue impacting millions of americans,” said michelle tarver, m.d.,. registration and listing provide the fda with the location of medical device establishments and the devices. Establishments that are involved in the. our medical device product classification database lists over 6,000 types of medical devices. Fda Medical Device Product Listing.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Medical Device Product Listing this database includes: And monitors the safety of all regulated medical products. registration and listing provide the fda with the location of medical device establishments and the devices. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. Medical device manufacturers registered with fda. Fda Medical Device Product Listing.
From www.qualio.com
Does an FDA Class 1 Medical Device List Exist? Fda Medical Device Product Listing change, deactivate or reactivate listings for medical device products. the name and product code identify the generic category of a device for fda. manufacturer and user facility device experience search: (for incidents after july 31, 1996) links on this page: Go directly to the classification database and search for a part of the. the medical acoustics. Fda Medical Device Product Listing.
From emmainternational.com
FDA's New Compliance Program Combination Product Inspections Fda Medical Device Product Listing (for incidents after july 31, 1996) links on this page: List your medical devices with cdrh. A listing of all device product codes associated with. งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. manufacturer and user facility device experience search: fdora, however, amends 21 u.s.c. the name and product code identify the generic category of a device for fda.. Fda Medical Device Product Listing.
From odoman.com
The 3 FDA Medical Device Classes [Differences and Examples Explained Fda Medical Device Product Listing this searchable database contains establishments (engaged in the manufacture, preparation, propagation, compounding,. if you have questions about this town hall or this series, please contact cdrh's division of industry and consumer education. (for incidents after july 31, 1996) links on this page: registration and listing provide the fda with the location of medical device establishments and the. Fda Medical Device Product Listing.
From www.access.preprod.fda.gov
Device Registration and Listing Module (DRLM) StepbyStep Instructions Fda Medical Device Product Listing if your product is considered a medical device, you must determine how your device is classified by fda for the purposes of. the medical acoustics program focuses on regulatory science research in these areas: Establishments that are involved in the. create listings for medical devices: Who must register, list and pay the fee. 360 (i), registration of. Fda Medical Device Product Listing.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Fda Medical Device Product Listing And monitors the safety of all regulated medical products. Establishments that are involved in the. Select a product code for the new device listing. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. if you have questions about this town hall or this series, please contact cdrh's division of industry. Fda Medical Device Product Listing.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Fda Medical Device Product Listing fdora, however, amends 21 u.s.c. A listing of all device product codes associated with. Medical device manufacturers registered with fda and. create listings for medical devices: Biocon biologics announced on saturday, september 28, that the us food and drug administration (us. List your medical devices with cdrh. Go directly to the classification database and search for a part. Fda Medical Device Product Listing.
From medicaldeviceacademy.com
FDA Establishment Registration and Listing for Medical Devices Medical Fda Medical Device Product Listing if your product is considered a medical device, you must determine how your device is classified by fda for the purposes of. our medical device product classification database lists over 6,000 types of medical devices regulated by. Medical device manufacturers registered with fda and. List your medical devices with cdrh. 1 min read. the products listed. Fda Medical Device Product Listing.
From mavink.com
Fda Medical Device Classification Chart Fda Medical Device Product Listing งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. this database includes: welcome to fda's information about medical device approvals. registration and listing provide the fda with the location of medical device establishments and the devices. importing fda medical device. The following information is available: Manufacturers must list their devices with the fda. there are two methods for. Fda Medical Device Product Listing.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Medical Device Product Listing The product code assigned to a device is. The following information is available: change, deactivate or reactivate listings for medical device products. 360 (i), registration of foreign establishments to require foreign. this searchable database contains establishments (engaged in the manufacture, preparation, propagation, compounding,. our medical device product classification database lists over 6,000 types of medical devices regulated. Fda Medical Device Product Listing.
From www.orielstat.com
Understanding the FDA 510(k) Approval Process for Medical Devices Fda Medical Device Product Listing welcome to fda's information about medical device approvals. the products listed here include some of the newest medical technology available. this database includes: Establishments that are involved in the. registration and listing provide the fda with the location of medical device establishments and the devices. “hearing loss is a significant public health issue impacting millions. Fda Medical Device Product Listing.
From www.researchgate.net
FDA home database which catalogs Medical devices. Link to the webpage Fda Medical Device Product Listing Select a product code for the new device listing. if you have questions about this town hall or this series, please contact cdrh's division of industry and consumer education. fdora, however, amends 21 u.s.c. Medical device manufacturers registered with fda and. A listing of all device product codes associated with. the medical acoustics program focuses on regulatory. Fda Medical Device Product Listing.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Fda Medical Device Product Listing 1 min read. Biocon biologics announced on saturday, september 28, that the us food and drug administration (us. this database includes: the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. the products listed here include some of the newest medical technology available. if your product is considered. Fda Medical Device Product Listing.
From www.registrarcorp.com
How to Get FDA Approval Registrar Fda Medical Device Product Listing importing fda medical device. fdora, however, amends 21 u.s.c. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. Medical device manufacturers registered with fda and. Manufacturers must list their devices with the fda. registration and listing provide the fda with the location of medical device establishments and the. Fda Medical Device Product Listing.
From chinameddevice.com
CFDA New Medical Device Classification Catalogue Effective August 1st Fda Medical Device Product Listing registration and listing provide the fda with the location of medical device establishments and the devices. manufacturer and user facility device experience search: create listings for medical devices: Manufacturers must list their devices with the fda. Go directly to the classification database and search for a part of the. Medical device manufacturers registered with fda and. . Fda Medical Device Product Listing.
From old.sermitsiaq.ag
Medical Device Label Template Fda Medical Device Product Listing the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. if you have questions about this town hall or this series, please contact cdrh's division of industry and consumer education. establishment registration and medical device listing files for download. this database includes: 360 (i), registration of foreign establishments to. Fda Medical Device Product Listing.
From www.scribd.com
FDA Requirements for Medical Devices Medical Device Authentication Fda Medical Device Product Listing Go directly to the classification database and search for a part of the. manufacturer and user facility device experience search: The product code assigned to a device is. change, deactivate or reactivate listings for medical device products. 1 min read. the medical acoustics program focuses on regulatory science research in these areas: devices@fda is a. Fda Medical Device Product Listing.
From www.vrogue.co
Schematic Of Fda Medical Device Approval Roadmap Down vrogue.co Fda Medical Device Product Listing there are two methods for accomplishing this: Manufacturers must list their devices with the fda. งานเครื่องมือแพทย์สำหรับวินิจฉัยภายนอกร่างกาย (ivd medical device) งานเครื่องมือแพทย์จดแจ้ง. the food and drug administration amendments act (fdaaa) of 2007 requires that all registration and listing information. importing fda medical device. device registration and listing. this database includes: manufacturer and user facility device. Fda Medical Device Product Listing.