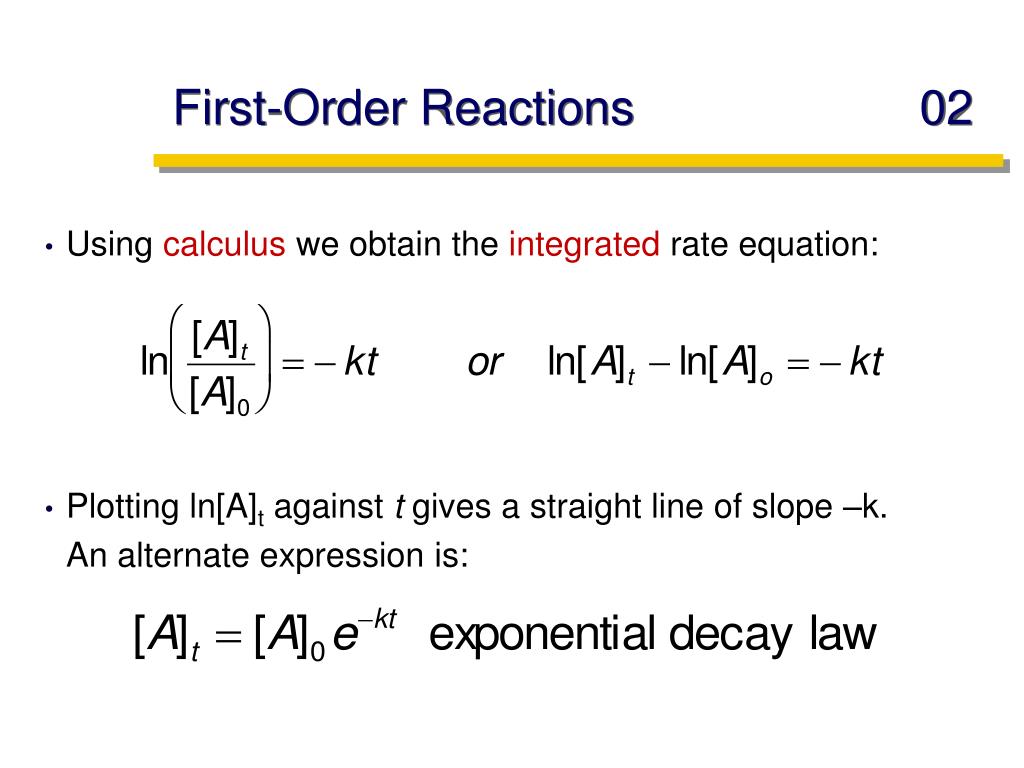
First Order Kinetics Equation . Find out what first order and second order reactions are, and how to calculate them using kinetic data. the adsorption kinetic data were analyzed using eight models in nonlinear forms: learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. chemical reactions may be assigned reaction orders that describe their kinetics. learn how to identify the reaction order from experimental data using differential and integrated rate laws. 1 kinetic order elimination equation, where delta [drug]. learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction rate. The types of orders are zero. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. As seen in equation no.
from
the adsorption kinetic data were analyzed using eight models in nonlinear forms: chemical reactions may be assigned reaction orders that describe their kinetics. As seen in equation no. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. 1 kinetic order elimination equation, where delta [drug]. The types of orders are zero. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. Find out what first order and second order reactions are, and how to calculate them using kinetic data.
First Order Kinetics Equation learn how to identify the reaction order from experimental data using differential and integrated rate laws. As seen in equation no. 1 kinetic order elimination equation, where delta [drug]. Find out what first order and second order reactions are, and how to calculate them using kinetic data. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. learn how to identify the reaction order from experimental data using differential and integrated rate laws. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. The types of orders are zero. chemical reactions may be assigned reaction orders that describe their kinetics. learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction rate. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. the adsorption kinetic data were analyzed using eight models in nonlinear forms:
From
First Order Kinetics Equation As seen in equation no. The types of orders are zero. chemical reactions may be assigned reaction orders that describe their kinetics. 1 kinetic order elimination equation, where delta [drug]. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. learn the definition, methods, and examples of. First Order Kinetics Equation.
From
First Order Kinetics Equation 1 kinetic order elimination equation, where delta [drug]. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. chemical reactions may be assigned reaction orders that describe their kinetics. As seen in equation no. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws,. First Order Kinetics Equation.
From www.youtube.com
integrated rate equation of first order reaction (chemical First Order Kinetics Equation learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. learn how to identify the reaction order from experimental data using differential and integrated rate laws. 1 kinetic order elimination equation, where delta [drug]. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department.. First Order Kinetics Equation.
From www.youtube.com
Integrated Rate Law First Order YouTube First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn how to identify the reaction order from experimental data using differential and integrated rate laws. As seen in equation no. 1 kinetic order elimination. First Order Kinetics Equation.
From www.slideshare.net
Chem 2 Chemical IV The FirstOrder Integrated Rate Law First Order Kinetics Equation As seen in equation no. learn how to identify the reaction order from experimental data using differential and integrated rate laws. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives,. First Order Kinetics Equation.
From
First Order Kinetics Equation learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. As seen in equation no. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. learn about reaction order, the power to which reactant concentrations appear in the. First Order Kinetics Equation.
From
First Order Kinetics Equation learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. the adsorption kinetic data were analyzed using eight models in nonlinear forms: The types of orders are. First Order Kinetics Equation.
From
First Order Kinetics Equation learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. 1 kinetic order elimination equation, where delta [drug]. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. the adsorption kinetic data were analyzed using eight models in nonlinear forms: As seen in equation. First Order Kinetics Equation.
From
First Order Kinetics Equation As seen in equation no. 1 kinetic order elimination equation, where delta [drug]. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn how to identify the reaction order from experimental data. First Order Kinetics Equation.
From
First Order Kinetics Equation The types of orders are zero. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. chemical reactions may be assigned reaction orders that describe their kinetics. the adsorption kinetic data were analyzed using eight models in nonlinear forms: As seen in equation no. 1 kinetic order. First Order Kinetics Equation.
From
First Order Kinetics Equation the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. 1 kinetic order elimination equation, where delta [drug]. learn how to identify the reaction order from experimental data using differential and integrated rate laws. As seen in equation no. chemical reactions may be assigned reaction orders that describe their kinetics. when discussing. First Order Kinetics Equation.
From
First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction. First Order Kinetics Equation.
From
First Order Kinetics Equation when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. The types of orders are zero. learn the definition, methods, and examples of first order kinetics, a. First Order Kinetics Equation.
From
First Order Kinetics Equation the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. Find out what first order and second order reactions are, and how to calculate them using kinetic data. learn how to identify the reaction order from experimental data using differential and integrated rate laws. As seen in equation no. when discussing kinetics, ''k''. First Order Kinetics Equation.
From www.youtube.com
YouTube First Order Kinetics Equation The types of orders are zero. learn how to identify the reaction order from experimental data using differential and integrated rate laws. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction. First Order Kinetics Equation.
From chemistnotes.com
First Order Reaction definition, example, half life period Chemistry First Order Kinetics Equation 1 kinetic order elimination equation, where delta [drug]. Find out what first order and second order reactions are, and how to calculate them using kinetic data. As seen in equation no. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. learn how to identify the reaction order from experimental data using differential and. First Order Kinetics Equation.
From www.biologyonline.com
Firstorder Definition and Examples Biology Online Dictionary First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: 1 kinetic order elimination equation, where delta [drug]. The types of orders are zero. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. As seen in equation no. learn how to approximate a 2nd. First Order Kinetics Equation.
From
First Order Kinetics Equation learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction rate. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. 1 kinetic order elimination equation, where delta [drug]. when discussing kinetics,. First Order Kinetics Equation.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. 1 kinetic order elimination equation, where delta [drug]. As seen in equation no. learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations. First Order Kinetics Equation.
From
First Order Kinetics Equation chemical reactions may be assigned reaction orders that describe their kinetics. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. Find out what first order and second. First Order Kinetics Equation.
From
First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. chemical reactions may be assigned reaction. First Order Kinetics Equation.
From
First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. learn how to identify the reaction order from experimental data using differential and integrated rate laws. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate. First Order Kinetics Equation.
From
First Order Kinetics Equation 1 kinetic order elimination equation, where delta [drug]. As seen in equation no. learn how to identify the reaction order from experimental data using differential and integrated rate laws. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. learn the basics of chemical reaction kinetics, including rate. First Order Kinetics Equation.
From www.youtube.com
Graph of first order integrated rate equation(chemical part 46 First Order Kinetics Equation learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the. First Order Kinetics Equation.
From thechemistrynotes.com
Differential and Integrated Rate Equation First Order Kinetics Equation The types of orders are zero. chemical reactions may be assigned reaction orders that describe their kinetics. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. As seen in equation no. learn about reaction order, the power to which reactant concentrations appear in the rate law,. First Order Kinetics Equation.
From
First Order Kinetics Equation the adsorption kinetic data were analyzed using eight models in nonlinear forms: learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. chemical reactions may be assigned. First Order Kinetics Equation.
From
First Order Kinetics Equation learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction rate. chemical reactions may be assigned reaction orders that describe their kinetics.. First Order Kinetics Equation.
From sharedocnow.blogspot.com
First Order Equation sharedoc First Order Kinetics Equation when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. Find out what first order and second order reactions are, and how to calculate them using kinetic data. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. learn the basics of chemical. First Order Kinetics Equation.
From ar.inspiredpencil.com
First Order Reaction Graph First Order Kinetics Equation when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction rate. learn how to approximate a 2nd order reaction as a 1st. First Order Kinetics Equation.
From
First Order Kinetics Equation learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. the adsorption kinetic data were analyzed using eight models in nonlinear forms: when discussing kinetics, ''k'' is the reaction constant, a value that relates the concentration of reactants to the rate of. learn how to identify the. First Order Kinetics Equation.
From
First Order Kinetics Equation learn how to approximate a 2nd order reaction as a 1st order reaction by manipulating the initial concentrations of. learn the basics of chemical reaction kinetics, including rate of reaction, rate laws, rate constants, half lives, and experimental techniques. 1 kinetic order elimination equation, where delta [drug]. the adsorption kinetic data were analyzed using eight models in. First Order Kinetics Equation.
From
First Order Kinetics Equation As seen in equation no. the adsorption kinetic data were analyzed using eight models in nonlinear forms: the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. learn the definition, methods, and examples of first order kinetics, a type of chemical reaction where the rate depends. chemical reactions may be assigned reaction. First Order Kinetics Equation.
From
First Order Kinetics Equation chemical reactions may be assigned reaction orders that describe their kinetics. The types of orders are zero. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. 1 kinetic order elimination equation, where delta [drug]. the adsorption kinetic data were analyzed using eight models in nonlinear forms: learn about reaction order, the. First Order Kinetics Equation.
From www.youtube.com
Pseudo first order model fitting in origin YouTube First Order Kinetics Equation learn how to identify the reaction order from experimental data using differential and integrated rate laws. the following pharmacological definition has been taken from the pharmacology and experimental therapeutics department. The types of orders are zero. As seen in equation no. learn about reaction order, the power to which reactant concentrations appear in the rate law, and. First Order Kinetics Equation.
From
First Order Kinetics Equation 1 kinetic order elimination equation, where delta [drug]. learn about reaction order, the power to which reactant concentrations appear in the rate law, and how it affects the rate constant and the reaction rate. the adsorption kinetic data were analyzed using eight models in nonlinear forms: learn how to identify the reaction order from experimental data using. First Order Kinetics Equation.