Why Only H2So4 Is Used In Kmno4 Titration . In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Find the theory, materials, procedure, observations, calculations and. Why is kmno4 used for titration? Hence sulfuric acid is stable in the present of strong oxidising. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur.
from www.chegg.com
Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Why is kmno4 used for titration? Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Find the theory, materials, procedure, observations, calculations and.
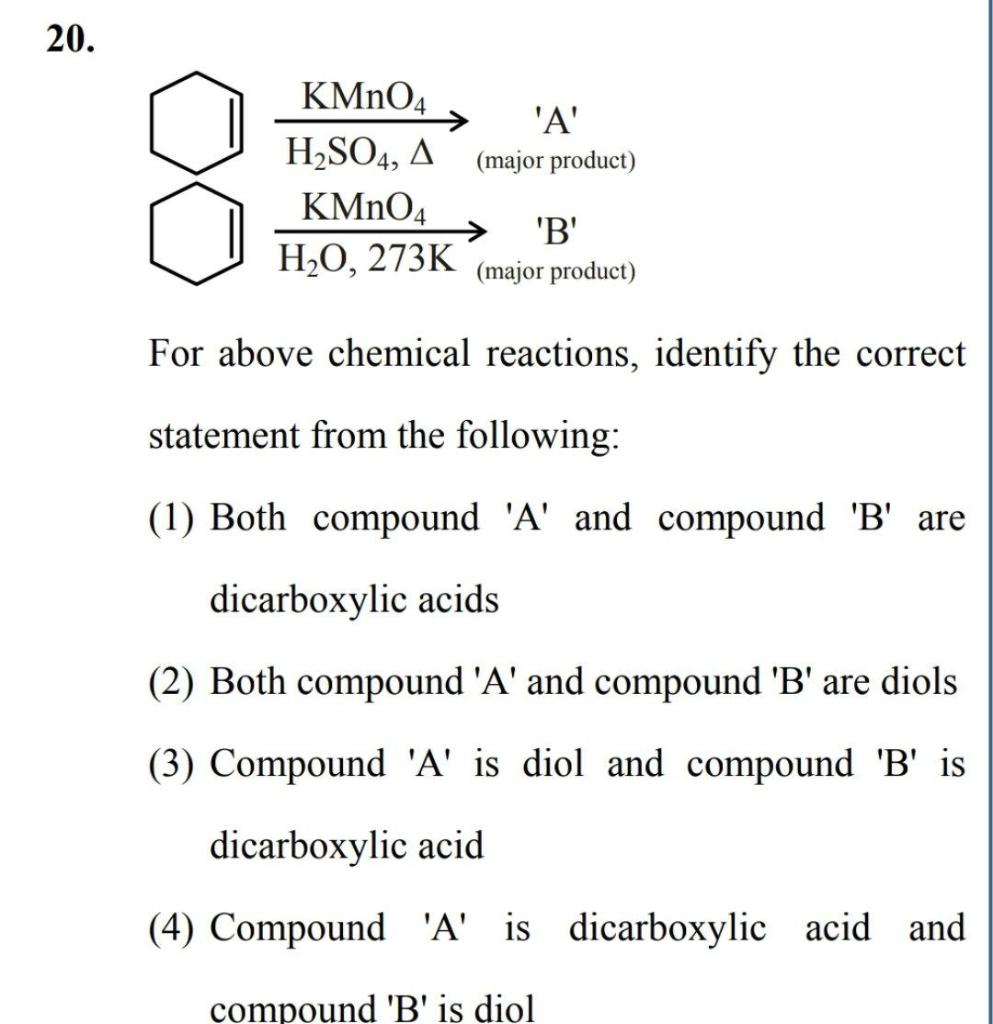
Solved 20. 'A' (major product) KMnO4 H2SO4, A KMnO4 H2O,
Why Only H2So4 Is Used In Kmno4 Titration Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hence sulfuric acid is stable in the present of strong oxidising. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Why is kmno4 used for titration? Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Find the theory, materials, procedure, observations, calculations and.
From www.meritnation.com
a) In the titration of FeSO4 with KMnO4 in the acidic medium, why is Why Only H2So4 Is Used In Kmno4 Titration Hence sulfuric acid is stable in the present of strong oxidising. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Find the theory, materials, procedure, observations, calculations and. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Why is kmno4 used for titration?. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
Action of Cold Conc H2SO4 on KMnO4 D and F Block Elements Chemistry Why Only H2So4 Is Used In Kmno4 Titration Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Why is kmno4 used for titration? Learn how to titrate potassium permanganate (kmno4) against oxalic. Why Only H2So4 Is Used In Kmno4 Titration.
From tuitiontube.com
KMnO4 + H2SO4 + Na2SO3 = K2SO4 + MnSO4 + Na2SO4 + H2O Tuition Tube Why Only H2So4 Is Used In Kmno4 Titration Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Find the theory, materials, procedure, observations, calculations and. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. H2so4 increase. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
Titration of KMnO4 and Oxalic Acid Charushila Choudhari YouTube Why Only H2So4 Is Used In Kmno4 Titration Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hydrochloric acid (hcl) is usually not used in. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
SOLVED *This is the oxidationreduction titration experiment.* EXP. A Why Only H2So4 Is Used In Kmno4 Titration Find the theory, materials, procedure, observations, calculations and. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hence sulfuric acid is stable in the present of strong oxidising. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator. Why Only H2So4 Is Used In Kmno4 Titration.
From www.transtutors.com
(Solved) Balance Chemical Equations! A) KMnO4 (Aq) + H2SO4 (Aq Why Only H2So4 Is Used In Kmno4 Titration Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Find the theory, materials, procedure, observations, calculations and. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Learn how to titrate potassium permanganate (kmno4) against. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
SOLVED Using the halfredox method, what is the balanced redox Why Only H2So4 Is Used In Kmno4 Titration Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hence sulfuric acid is stable in the present of strong oxidising. Find the theory, materials, procedure, observations, calculations and. In the redox titration. Why Only H2So4 Is Used In Kmno4 Titration.
From www.scribd.com
Why We Use H2so4 in KMnO4 Titration PDF Titration Chemistry Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Hence sulfuric acid is stable in the present of strong oxidising. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hydrochloric acid (hcl) is usually not used. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Learn how to. Why Only H2So4 Is Used In Kmno4 Titration.
From www.scribd.com
Titration of Oxalic Acid With KMnO4 PDF Why Only H2So4 Is Used In Kmno4 Titration Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hence sulfuric acid is stable in the present of strong oxidising. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the. Why Only H2So4 Is Used In Kmno4 Titration.
From www.toppr.com
Hydrogen peroxide solution ( 20 mL) reacts quantitatively with a Why Only H2So4 Is Used In Kmno4 Titration Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Why is kmno4 used for titration? Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Hydrochloric acid. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
How to Balance the H2S+KMnO4=MnO2+H2SO4+K Chemical Equation YouTube Why Only H2So4 Is Used In Kmno4 Titration Hence sulfuric acid is stable in the present of strong oxidising. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). In the redox titration of $\ce{feso4}$ with. Why Only H2So4 Is Used In Kmno4 Titration.
From www.chegg.com
Solved 20. 'A' (major product) KMnO4 H2SO4, A KMnO4 H2O, Why Only H2So4 Is Used In Kmno4 Titration Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Find the theory, materials, procedure, observations, calculations and. Hence sulfuric acid is stable in the present of strong oxidising. In the redox titration. Why Only H2So4 Is Used In Kmno4 Titration.
From www.toppr.com
Complete and balance the following chemical equations(i) KMnO4 + KI Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Find the theory, materials, procedure,. Why Only H2So4 Is Used In Kmno4 Titration.
From www.congress-intercultural.eu
To Determine The Molarity Of KMnO4 Solution By Titrating It, 44 OFF Why Only H2So4 Is Used In Kmno4 Titration Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Sulphuric acid (h2so4) is used in the redox. Why Only H2So4 Is Used In Kmno4 Titration.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Find the theory, materials, procedure, observations, calculations and. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Hence sulfuric acid is stable in the present of strong oxidising. Learn how to. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
A solution of sodium oxalate (Na2C2O4) in acidic solution is titrated Why Only H2So4 Is Used In Kmno4 Titration Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hence sulfuric acid is stable in the present of strong oxidising. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
KMnO4+H2SO4=K2SO4+MnSO4+H2O+O2 balance the chemical equation Why Only H2So4 Is Used In Kmno4 Titration In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Why is kmno4 used for titration? Find the theory, materials, procedure, observations, calculations and. Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hence sulfuric acid is stable in the present of strong oxidising. Sulphuric. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of KMnO4 Why Only H2So4 Is Used In Kmno4 Titration Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Hence sulfuric acid is stable in the present of strong oxidising. Hydrochloric. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Why Only H2So4 Is Used In Kmno4 Titration Find the theory, materials, procedure, observations, calculations and. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Why is kmno4 used for titration? Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Learn how. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
SOLVED The concentration of a potassium permanganate (KMnO4) solution Why Only H2So4 Is Used In Kmno4 Titration Why is kmno4 used for titration? Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Kmno4 (potassium permanganate) is a strong oxidizing agent that can. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
WHY HCl IS NOT USED WITH KMnO4 INDICATOR AND H2SO4 IS USED youtube Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Why is kmno4 used for titration? Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment Why Only H2So4 Is Used In Kmno4 Titration In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Find the theory, materials, procedure, observations, calculations and. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hydrochloric acid (hcl) is usually not used in the process. Why Only H2So4 Is Used In Kmno4 Titration.
From moreref.com
Why Sulphuric acid is used in KMnO4 titration? More REF Why Only H2So4 Is Used In Kmno4 Titration Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Find the theory, materials, procedure, observations, calculations and. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is.. Why Only H2So4 Is Used In Kmno4 Titration.
From tuitiontube.com
Balancing CaC2O4 + H2SO4 + KMnO4 = K2SO4 + CaSO4 + MnSO4 + H2O + CO2 Why Only H2So4 Is Used In Kmno4 Titration Why is kmno4 used for titration? Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Hence sulfuric acid is stable in the present of strong oxidising. Find the theory, materials, procedure, observations, calculations. Why Only H2So4 Is Used In Kmno4 Titration.
From www.chegg.com
Solved Sodium oxalate can be titrated with KMnO4 under Why Only H2So4 Is Used In Kmno4 Titration Why is kmno4 used for titration? In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Find the theory, materials, procedure, observations, calculations and. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hence sulfuric acid is. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
PROBLEM 1. A solution of H2O2 when titrated against KMnO4 solution at Why Only H2So4 Is Used In Kmno4 Titration Find the theory, materials, procedure, observations, calculations and. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Why is kmno4 used for titration? Kmno4 (potassium permanganate) is a strong. Why Only H2So4 Is Used In Kmno4 Titration.
From www.reddit.com
Why does the FeSO4 (added with H2SO4) titrated with KMnO4 slowly Why Only H2So4 Is Used In Kmno4 Titration In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Why is kmno4 used for titration? Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Learn how to titrate potassium. Why Only H2So4 Is Used In Kmno4 Titration.
From tuitiontube.com
NaCl + KMnO4 + H2SO4 = Cl2 + MnSO4 + Na2SO4 + K2SO4 + H2O Tuition Tube Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Why is kmno4 used for titration? In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Hence sulfuric acid is stable in the present of strong oxidising. Find the theory, materials, procedure,. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
Calculation of KMnO4 and Oxalic acid titration YouTube Why Only H2So4 Is Used In Kmno4 Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Why is kmno4 used for titration? Kmno4 (potassium permanganate) is a strong oxidizing agent that can react with a wide range. Find the theory, materials, procedure, observations, calculations and. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in. Why Only H2So4 Is Used In Kmno4 Titration.
From www.slideserve.com
PPT REDOX TITRATIONS PowerPoint Presentation ID2670469 Why Only H2So4 Is Used In Kmno4 Titration Why is kmno4 used for titration? Find the theory, materials, procedure, observations, calculations and. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Kmno4 (potassium permanganate) is a strong oxidizing agent that. Why Only H2So4 Is Used In Kmno4 Titration.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Why Only H2So4 Is Used In Kmno4 Titration Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Sulphuric acid (h2so4) is used in the redox. Why Only H2So4 Is Used In Kmno4 Titration.
From www.numerade.com
SOLVED 13. In a volumetric analysis (redox titration) experiment, a Why Only H2So4 Is Used In Kmno4 Titration Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Find the theory, materials, procedure, observations, calculations and. Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of. Why Only H2So4 Is Used In Kmno4 Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Why Only H2So4 Is Used In Kmno4 Titration Why is kmno4 used for titration? In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hence sulfuric acid is stable in the present of strong oxidising. Find. Why Only H2So4 Is Used In Kmno4 Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Why Only H2So4 Is Used In Kmno4 Titration Sulphuric acid (h2so4) is used in the redox titration process because it provides the h (+) ions necessary for the reaction to occur. Hydrochloric acid (hcl) is usually not used in the process of titration because it reacts with the indicator potassium permanganate (kmno4). Learn how to titrate potassium permanganate (kmno4) against oxalic acid (c2h2o4) in acidic medium. In the. Why Only H2So4 Is Used In Kmno4 Titration.