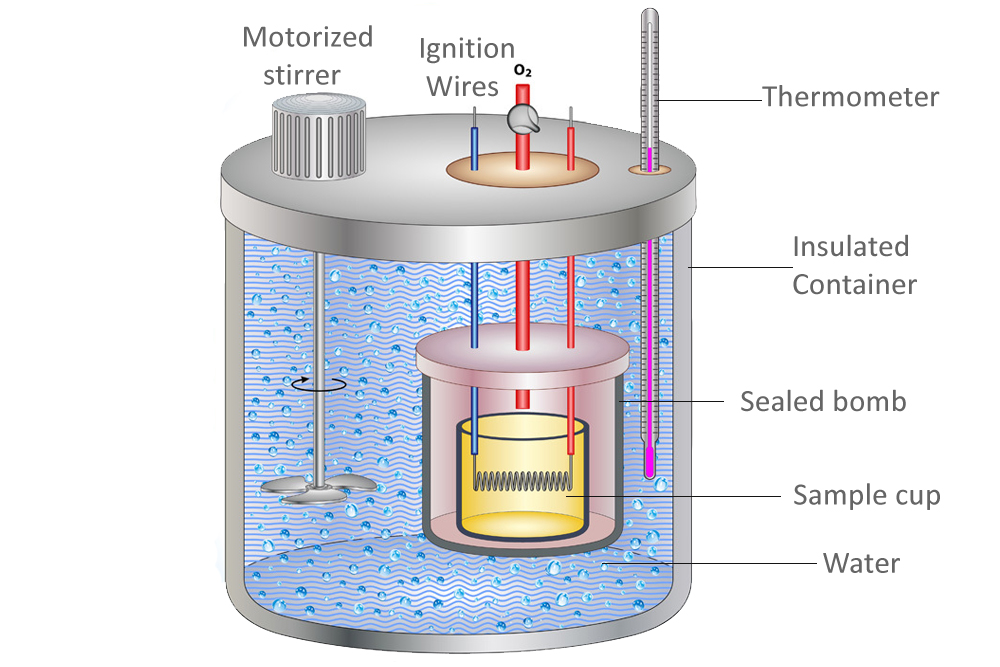
Information About Calorimeter . Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is what is used to measure the thermal changes of a body. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. what is calorimetry? calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. apply the first law of thermodynamics to calorimetry. Calorimetry is applied extensively in the fields of thermochemistry in.
from www.scienceabc.com
calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. apply the first law of thermodynamics to calorimetry. Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is what is used to measure the thermal changes of a body. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. what is calorimetry? a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
Molar Heat Capacity Definition, Formula, Equation, Calculation
Information About Calorimeter what is calorimetry? apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is what is used to measure the thermal changes of a body. Calorimetry is applied extensively in the fields of thermochemistry in. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to.
From courses.lumenlearning.com
Calorimetry Chemistry I Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimetry is applied extensively in the. Information About Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry Information About Calorimeter what is calorimetry? calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. apply the first law of thermodynamics to calorimetry. a calorimeter is what is used to measure the thermal changes of a body. Calorimetry is a branch of science concerned with measuring a body’s state in. Information About Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Information About Calorimeter a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimetry is applied extensively in the fields of thermochemistry in. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. what is calorimetry? Compare heat flow from. Information About Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Information About Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what is calorimetry? a calorimeter is what is used to measure the thermal changes of a body. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is a method of. Information About Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is what is used to measure the thermal changes of a body. a calorimeter. Information About Calorimeter.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Information About Calorimeter what is calorimetry? Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. apply the first law of thermodynamics to calorimetry. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes,. Information About Calorimeter.
From study.com
Calorimetry Definition, Equation & Types Lesson Information About Calorimeter apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is what is. Information About Calorimeter.
From physique.ensc-rennes.fr
TP calorimetry Information About Calorimeter a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is what is. Information About Calorimeter.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Information About Calorimeter a calorimeter is what is used to measure the thermal changes of a body. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. what is calorimetry? Calorimetry is applied extensively. Information About Calorimeter.
From www.youtube.com
Learn about Calorimeter/ Calorimetry/Physics(Heat) 🔥 YouTube Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimetry is applied extensively in the fields of thermochemistry in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is what is used to measure the thermal changes of. Information About Calorimeter.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Information About Calorimeter apply the first law of thermodynamics to calorimetry. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimetry is a branch of science concerned with measuring a body’s state in. Information About Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal. Information About Calorimeter.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Information About Calorimeter what is calorimetry? Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is applied extensively in the fields of thermochemistry in. apply the first law of thermodynamics to calorimetry. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimetry is. Information About Calorimeter.
From ecampusontario.pressbooks.pub
3.5 Calorimétrie La Chimie Générale pour les GeeGees Information About Calorimeter what is calorimetry? a calorimeter is what is used to measure the thermal changes of a body. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Compare heat flow. Information About Calorimeter.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Information About Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. apply the first law of thermodynamics to calorimetry. what is calorimetry? a calorimeter is what is used to measure the thermal changes of. Information About Calorimeter.
From www.youtube.com
050 Calorimetry YouTube Information About Calorimeter Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is what is used to measure. Information About Calorimeter.
From www.slideserve.com
PPT Lab Procedures and Techniques PowerPoint Presentation, free Information About Calorimeter Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is what is used to measure the thermal changes of a body. apply the first law of thermodynamics to calorimetry. what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimeter, device for. Information About Calorimeter.
From users.highland.edu
Calorimetry Information About Calorimeter calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. apply the first law of thermodynamics to calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Compare heat flow from hot to cold objects in an. Information About Calorimeter.
From chem.libretexts.org
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions Information About Calorimeter a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is what is used to measure the thermal changes of a body. what is calorimetry? Calorimetry is applied extensively in the fields of thermochemistry in. calorimetry is a method of measuring the heat transfer within a. Information About Calorimeter.
From kaffee.50webs.com
Lab Calorimetry Information About Calorimeter a calorimeter is what is used to measure the thermal changes of a body. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is applied extensively in the fields of thermochemistry in. Calorimetry is a branch of science concerned with measuring a. Information About Calorimeter.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Information About Calorimeter a calorimeter is what is used to measure the thermal changes of a body. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. . Information About Calorimeter.
From www.pinterest.com
Calorimeter An object used for measuring the heat of chemical Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. apply the first law of thermodynamics to calorimetry. Calorimetry is a. Information About Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Information About Calorimeter apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. what is calorimetry? Compare heat flow from hot. Information About Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms. Information About Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between.. Information About Calorimeter.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Information About Calorimeter Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what is. Information About Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Information About Calorimeter a calorimeter is what is used to measure the thermal changes of a body. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. apply the first law of thermodynamics to calorimetry. a. Information About Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Information About Calorimeter what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is. Information About Calorimeter.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Information About Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical. Information About Calorimeter.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure Information About Calorimeter apply the first law of thermodynamics to calorimetry. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. Calorimetry is applied extensively in the fields of thermochemistry in. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what. Information About Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Information About Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. apply the first law of. Information About Calorimeter.
From hxehqxhcm.blob.core.windows.net
Lab Calorimetry And Specific Heat Student Guide at Bryan Arnold blog Information About Calorimeter Calorimetry is applied extensively in the fields of thermochemistry in. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is what is used to. Information About Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Information About Calorimeter calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. what is calorimetry? apply the first law of thermodynamics to calorimetry. a calorimeter is what is used to measure the thermal changes of a body. calorimeter, device for measuring the heat developed during. Information About Calorimeter.
From www.slideserve.com
PPT An introduction to calorimeters for particle physics PowerPoint Information About Calorimeter a calorimeter is what is used to measure the thermal changes of a body. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. apply the. Information About Calorimeter.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Information About Calorimeter Calorimetry is applied extensively in the fields of thermochemistry in. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. apply the first law of thermodynamics to calorimetry. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change. Information About Calorimeter.