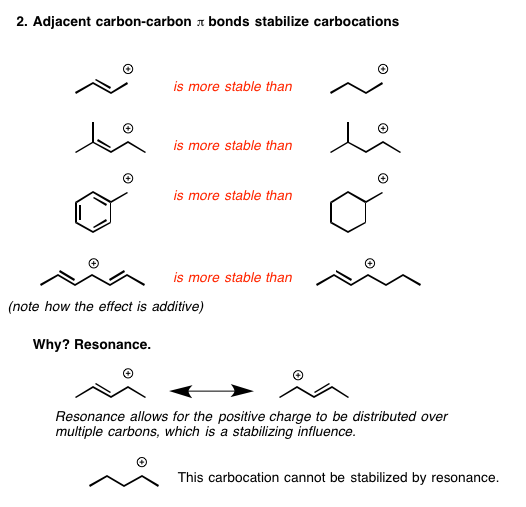
Stabilization Of Carbocation . Conversely, a carbocation will be destabilized by an electron withdrawing group. Imagine a tightrope walker (a carbocation) trying to maintain balance. Interaction of neighboring c−h σ bonds with the vacant p. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Conversely, a carbocation will be. Three main factors increase the stability of carbocations: Arrange a given series of carbocations in order of increasing or decreasing stability. The critical question now becomes, what stabilizes a carbocation? Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Increasing the number of adjacent carbon.
from socratic.org
Conversely, a carbocation will be destabilized by an electron withdrawing group. Arrange a given series of carbocations in order of increasing or decreasing stability. Interaction of neighboring c−h σ bonds with the vacant p. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. The critical question now becomes, what stabilizes a carbocation? Three main factors increase the stability of carbocations: Increasing the number of adjacent carbon. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be.
What stabilizes carbocation? Socratic
Stabilization Of Carbocation Imagine a tightrope walker (a carbocation) trying to maintain balance. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be destabilized by an electron withdrawing group. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Conversely, a carbocation will be. The critical question now becomes, what stabilizes a carbocation? Interaction of neighboring c−h σ bonds with the vacant p. Arrange a given series of carbocations in order of increasing or decreasing stability. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Three main factors increase the stability of carbocations: Increasing the number of adjacent carbon.
From www.organicchemistrytutor.com
Carbocations Stability and Rearrangements — Organic Chemistry Tutor Stabilization Of Carbocation Conversely, a carbocation will be destabilized by an electron withdrawing group. The critical question now becomes, what stabilizes a carbocation? Imagine a tightrope walker (a carbocation) trying to maintain balance. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms.. Stabilization Of Carbocation.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID3535377 Stabilization Of Carbocation Three main factors increase the stability of carbocations: Interaction of neighboring c−h σ bonds with the vacant p. Conversely, a carbocation will be destabilized by an electron withdrawing group. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. The critical. Stabilization Of Carbocation.
From www.vedantu.com
The stability order of carbocation is\n \n \n \n \n (A) II IV III I(B Stabilization Of Carbocation Conversely, a carbocation will be destabilized by an electron withdrawing group. Conversely, a carbocation will be. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Increasing the number of adjacent carbon. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Interaction of neighboring c−h σ bonds with. Stabilization Of Carbocation.
From www.youtube.com
Stability of carbocations, radicals, and carboanions YouTube Stabilization Of Carbocation Increasing the number of adjacent carbon. Three main factors increase the stability of carbocations: The critical question now becomes, what stabilizes a carbocation? Conversely, a carbocation will be. Imagine a tightrope walker (a carbocation) trying to maintain balance. Interaction of neighboring c−h σ bonds with the vacant p. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in. Stabilization Of Carbocation.
From www.organicchemistrytutor.com
Carbocations Stability and Rearrangements — Organic Chemistry Tutor Stabilization Of Carbocation Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Interaction of neighboring c−h σ bonds with the vacant p. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be destabilized by an electron withdrawing. Stabilization Of Carbocation.
From www.clutchprep.com
Rank the following carbocations in order of decreasing stability Stabilization Of Carbocation Three main factors increase the stability of carbocations: Imagine a tightrope walker (a carbocation) trying to maintain balance. Arrange a given series of carbocations in order of increasing or decreasing stability. Interaction of neighboring c−h σ bonds with the vacant p. Conversely, a carbocation will be. Conversely, a carbocation will be destabilized by an electron withdrawing group. Figure 7.13 stabilization. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Interaction of neighboring c−h σ bonds with the vacant p. Increasing the number of adjacent carbon. Conversely, a carbocation will be destabilized by an electron withdrawing group. Arrange a given series of carbocations in order of increasing or decreasing stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. The critical question now becomes, what stabilizes. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Interaction of neighboring c−h σ bonds with the vacant p. Conversely, a carbocation will be destabilized by an electron withdrawing group. Three main factors increase the stability of carbocations: Imagine a tightrope walker (a carbocation) trying to maintain balance. Arrange a given series of carbocations in order of increasing or decreasing stability. Conversely, a carbocation will be. Figure 7.13 stabilization. Stabilization Of Carbocation.
From www.youtube.com
Stability of Carbocation trends Stability of Carbocations Organic Stabilization Of Carbocation Increasing the number of adjacent carbon. Imagine a tightrope walker (a carbocation) trying to maintain balance. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. The critical question now becomes, what stabilizes a carbocation? Three main factors increase the stability of carbocations: Interaction of neighboring c−h σ bonds with the vacant p. Conversely, a carbocation. Stabilization Of Carbocation.
From www.youtube.com
Carbocation Stability Hyperconjugation, Inductive Effect & Resonance Stabilization Of Carbocation Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Conversely, a carbocation will be destabilized by an electron withdrawing group. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be. Three main factors increase the stability of carbocations: Carbocations are stabilized when they are adjacent to elements with electron pairs. Stabilization Of Carbocation.
From trickychemistrysuman.blogspot.com
Stability of carbocation a detailed explanation DPO Udham Singh Nagar Stabilization Of Carbocation Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Imagine a tightrope walker (a carbocation) trying to maintain balance. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. The critical question now becomes, what stabilizes a carbocation? Interaction of neighboring c−h σ bonds with the vacant p. Increasing. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Increasing the number of adjacent carbon. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Conversely, a carbocation will be. Interaction of neighboring c−h σ bonds with the vacant p. Imagine a tightrope walker (a carbocation) trying to. Stabilization Of Carbocation.
From slideplayer.com
Structure, Bonding, and Stability of Carbocations ppt download Stabilization Of Carbocation Arrange a given series of carbocations in order of increasing or decreasing stability. The critical question now becomes, what stabilizes a carbocation? Interaction of neighboring c−h σ bonds with the vacant p. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be destabilized by an electron withdrawing group. Carbocations are stabilized when they are adjacent. Stabilization Of Carbocation.
From thechemistrynotes.com
Carbocation Stability Definition, Factors, Order Stabilization Of Carbocation Interaction of neighboring c−h σ bonds with the vacant p. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. The critical question now becomes, what stabilizes a carbocation? Imagine a tightrope walker (a carbocation) trying to maintain balance. Increasing the number of adjacent carbon. Three main factors increase the stability of carbocations: Carbocations are stabilized when. Stabilization Of Carbocation.
From www.doubtnut.com
'Stability of carbocations depends upon the electron releasing inducti Stabilization Of Carbocation Arrange a given series of carbocations in order of increasing or decreasing stability. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Interaction of neighboring c−h σ bonds with the vacant p. Conversely, a carbocation will be. Imagine a tightrope walker (a carbocation) trying to maintain balance. Increasing the number of adjacent carbon. Explain the. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be. Increasing the number of adjacent carbon. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Three main factors increase the stability of carbocations: The critical question now becomes, what stabilizes a carbocation? Carbocations are stabilized when they are adjacent to elements. Stabilization Of Carbocation.
From courses.lumenlearning.com
Carbocation Structure and Stability MCC Organic Chemistry Stabilization Of Carbocation The critical question now becomes, what stabilizes a carbocation? Conversely, a carbocation will be. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Three main factors increase the stability of carbocations: Arrange a given series of carbocations in. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations — Master Organic Chemistry Stabilization Of Carbocation Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Three main factors increase the stability of carbocations: The critical question now becomes, what stabilizes a carbocation? Conversely, a carbocation will be. Imagine a tightrope walker (a carbocation) trying to maintain balance. Explain the relative stability of methyl, primary, secondary and tertiary carbocations. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation The critical question now becomes, what stabilizes a carbocation? Increasing the number of adjacent carbon. Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be destabilized by an electron withdrawing group. Interaction of neighboring c−h σ bonds with the vacant p. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation.. Stabilization Of Carbocation.
From www.organicchemistrytutor.com
Carbocations Stability and Rearrangements — Organic Chemistry Tutor Stabilization Of Carbocation Conversely, a carbocation will be. Increasing the number of adjacent carbon. Imagine a tightrope walker (a carbocation) trying to maintain balance. Arrange a given series of carbocations in order of increasing or decreasing stability. Conversely, a carbocation will be destabilized by an electron withdrawing group. Three main factors increase the stability of carbocations: The critical question now becomes, what stabilizes. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Arrange a given series of carbocations in order of increasing or decreasing stability. Conversely, a carbocation will be. The critical question now becomes, what stabilizes a carbocation? Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Carbocations are stabilized when they are. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Increasing the number of adjacent carbon. Three main factors increase the stability of carbocations: Imagine a tightrope walker (a carbocation) trying to maintain balance. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Conversely, a carbocation will be. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Conversely, a carbocation will. Stabilization Of Carbocation.
From chem-net.blogspot.com
Carbocations Factors affecting their Stability Chemistry Net Stabilization Of Carbocation Imagine a tightrope walker (a carbocation) trying to maintain balance. Conversely, a carbocation will be destabilized by an electron withdrawing group. Increasing the number of adjacent carbon. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Arrange a given series of carbocations in order of increasing or decreasing stability. Carbocations are stabilized when they are. Stabilization Of Carbocation.
From www.pinterest.com
Carbocation Stability Primary Secondary Tertiary Allylic and Benzylic Stabilization Of Carbocation Three main factors increase the stability of carbocations: Conversely, a carbocation will be destabilized by an electron withdrawing group. Imagine a tightrope walker (a carbocation) trying to maintain balance. Interaction of neighboring c−h σ bonds with the vacant p. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Figure 7.13 stabilization of. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Arrange a given series of carbocations in order of increasing or decreasing stability. Interaction of neighboring c−h σ bonds with the vacant p. Three main factors increase the stability of carbocations: The critical question now becomes, what stabilizes a carbocation? Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Increasing the number. Stabilization Of Carbocation.
From www.aceorganicchem.com
Carbocation Stability [with free study guide] organic chemistry help Stabilization Of Carbocation Interaction of neighboring c−h σ bonds with the vacant p. The critical question now becomes, what stabilizes a carbocation? Increasing the number of adjacent carbon. Imagine a tightrope walker (a carbocation) trying to maintain balance. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Arrange a given series of carbocations in order of increasing or. Stabilization Of Carbocation.
From leah4sci.com
Carbocation Stability and Ranking Organic Chemistry Tutorial Stabilization Of Carbocation Three main factors increase the stability of carbocations: Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Conversely, a carbocation will be destabilized by an electron withdrawing group. Imagine a tightrope walker (a carbocation) trying to maintain balance.. Stabilization Of Carbocation.
From socratic.org
What stabilizes carbocation? Socratic Stabilization Of Carbocation Conversely, a carbocation will be. Three main factors increase the stability of carbocations: Conversely, a carbocation will be destabilized by an electron withdrawing group. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Increasing the number of adjacent carbon. Interaction of neighboring c−h σ bonds with the vacant p. Figure 7.13 stabilization of the ethyl carbocation,. Stabilization Of Carbocation.
From socratic.org
What determines carbocation stability? + Example Stabilization Of Carbocation Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Conversely, a carbocation will be destabilized by an electron withdrawing group. Increasing the number of adjacent carbon. Imagine a tightrope walker (a carbocation) trying to maintain balance. Three main factors increase the stability of carbocations: Arrange a given series of carbocations in order. Stabilization Of Carbocation.
From courses.lumenlearning.com
Carbocation Structure and Stability MCC Organic Chemistry Stabilization Of Carbocation Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Three main factors increase the stability of carbocations: Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Imagine a tightrope walker (a carbocation) trying to. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Three main factors increase the stability of carbocations: Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Increasing the number of adjacent carbon. Interaction of neighboring c−h σ bonds with the vacant p. Conversely, a carbocation will be. The critical question now. Stabilization Of Carbocation.
From www.youtube.com
Carbocation Stability YouTube Stabilization Of Carbocation Interaction of neighboring c−h σ bonds with the vacant p. Increasing the number of adjacent carbon. The critical question now becomes, what stabilizes a carbocation? Arrange a given series of carbocations in order of increasing or decreasing stability. Conversely, a carbocation will be. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Conversely, a carbocation will. Stabilization Of Carbocation.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stabilization Of Carbocation Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Imagine a tightrope walker (a carbocation) trying to maintain balance. Three main factors increase the stability. Stabilization Of Carbocation.
From www.doubtnut.com
'Stability of carbocations depends upon the electron releasing inducti Stabilization Of Carbocation Figure 7.13 stabilization of the ethyl carbocation, ch 3 ch 2 +, through hyperconjugation. Carbocations are stabilized when they are adjacent to elements with electron pairs like oxygen, nitrogen, or sulfur. The critical question now becomes, what stabilizes a carbocation? Increasing the number of adjacent carbon. Interaction of neighboring c−h σ bonds with the vacant p. Imagine a tightrope walker. Stabilization Of Carbocation.
From www.organicchemistrytutor.com
Carbocations Stability and Rearrangements — Organic Chemistry Tutor Stabilization Of Carbocation Conversely, a carbocation will be. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms. Interaction of neighboring c−h σ bonds with the vacant p. The critical question now becomes, what stabilizes a carbocation? Arrange a given series of carbocations in order of increasing or decreasing stability. Conversely, a carbocation will be destabilized by an electron withdrawing. Stabilization Of Carbocation.