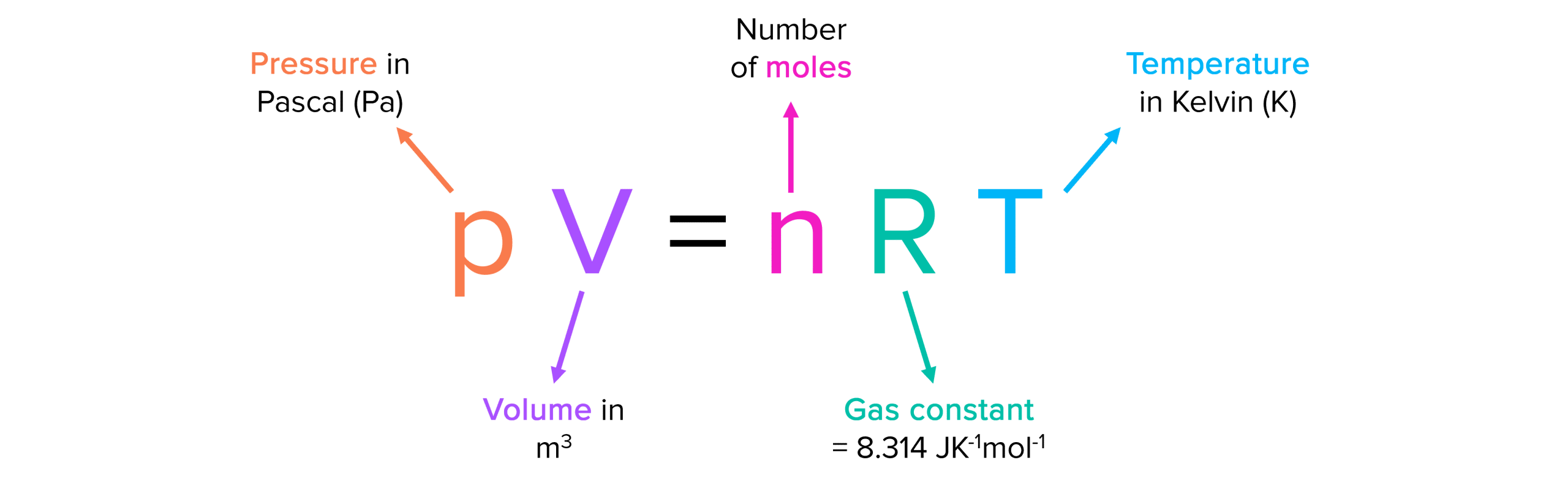What Is Ideal Gas Law . Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas law describes the behavior of real gases under most conditions. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume.
from mmerevise.co.uk
The ideal gas law describes the behavior of real gases under most conditions. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas.
The Ideal Gas Equation MME
What Is Ideal Gas Law The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas equation is valid as long as the density is low. The ideal gas law describes the behavior of real gases under most conditions. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law.
From sciencecalx.com
Ideal Gas Law Calculator ScienceCalx What Is Ideal Gas Law Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The ideal gas law describes the behavior of real gases under most conditions. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas equation is valid as long. What Is Ideal Gas Law.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of.. What Is Ideal Gas Law.
From www.youtube.com
Introduction to the Ideal Gas Law Formula YouTube What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of.. What Is Ideal Gas Law.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas.. What Is Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. The ideal gas law describes the behavior of real gases under most conditions. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law.. What Is Ideal Gas Law.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas. What Is Ideal Gas Law.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID What Is Ideal Gas Law The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The ideal gas equation is valid as long as the density is low. Boyle’s law describes the inverse proportional relationship between. What Is Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The kinetic energy. What Is Ideal Gas Law.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas law describes the behavior of real gases under most conditions.. What Is Ideal Gas Law.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas law describes the behavior of real gases under most conditions. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The kinetic energy. What Is Ideal Gas Law.
From studylib.net
Ideal Gas Law What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas law describes the behavior of real gases under most conditions. Explanation of the ideal gas law, including its formula and how it relates. What Is Ideal Gas Law.
From general.chemistrysteps.com
The Ideal Gas Law Chemistry Steps What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. The ideal gas law describes the behavior of real gases under most conditions. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and. What Is Ideal Gas Law.
From mmerevise.co.uk
The Ideal Gas Equation MME What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of.. What Is Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID What Is Ideal Gas Law The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law.. What Is Ideal Gas Law.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Boyle’s law describes. What Is Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. The ideal gas law describes the behavior of real gases under most conditions. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. Explanation of the ideal gas. What Is Ideal Gas Law.
From chemistryguru.com.sg
Ideal Gas Law and Applications What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas.. What Is Ideal Gas Law.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas.. What Is Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The ideal gas law describes the behavior of real gases under most conditions. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure,. What Is Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. Ideal gas, a gas that. What Is Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and. What Is Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 What Is Ideal Gas Law The ideal gas law describes the behavior of real gases under most conditions. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas.. What Is Ideal Gas Law.
From chemistryguru.com.sg
Ideal Gas Law and Applications What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas law describes the behavior of real gases under most conditions. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The kinetic energy. What Is Ideal Gas Law.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas law describes the behavior of real gases under most conditions. Ideal gas, a gas that. What Is Ideal Gas Law.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Explanation of the ideal gas law, including its formula and how it relates. What Is Ideal Gas Law.
From www.youtube.com
Ideal Gas Law Physics Problems With Boltzmann's Constant YouTube What Is Ideal Gas Law The ideal gas equation is valid as long as the density is low. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law.. What Is Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between. What Is Ideal Gas Law.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. The ideal gas law describes the behavior of real gases under most conditions. The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized. What Is Ideal Gas Law.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas law describes the behavior of real gases under most conditions. The ideal gas equation is valid as long as the density is low. Boyle’s law describes the inverse proportional relationship between. What Is Ideal Gas Law.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica What Is Ideal Gas Law Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The kinetic energy of the molecules greatly exceeds any potential energy of attraction. What Is Ideal Gas Law.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas equation is valid as long as the density is low.. What Is Ideal Gas Law.
From www.atmo.arizona.edu
Lecture 6 Ideal gas law, rising and sinking air What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The ideal gas equation is valid as long as the density is low. The ideal gas. What Is Ideal Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What Is Ideal Gas Law Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized relation between pressure, volume, and temperature called the ideal, or general, gas law. The ideal gas equation is valid as long as the density is low. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas.. What Is Ideal Gas Law.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With What Is Ideal Gas Law The ideal gas law describes the behavior of real gases under most conditions. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. The ideal gas equation is valid as long as the density is low. Ideal gas, a gas that conforms, in physical behaviour, to a particular idealized. What Is Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Is Ideal Gas Law The ideal gas law describes the behavior of real gases under most conditions. Explanation of the ideal gas law, including its formula and how it relates to temperature, pressure, and volume. The kinetic energy of the molecules greatly exceeds any potential energy of attraction or repulsion between molecules and the size of. The ideal gas equation is valid as long. What Is Ideal Gas Law.