Titration Basic Equation . A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Calculate the amount of sodium hydroxide in moles. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Calculate the ph at these.
from www.tes.com
Calculate the amount of sodium hydroxide in moles. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Calculate the ph at these. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]).
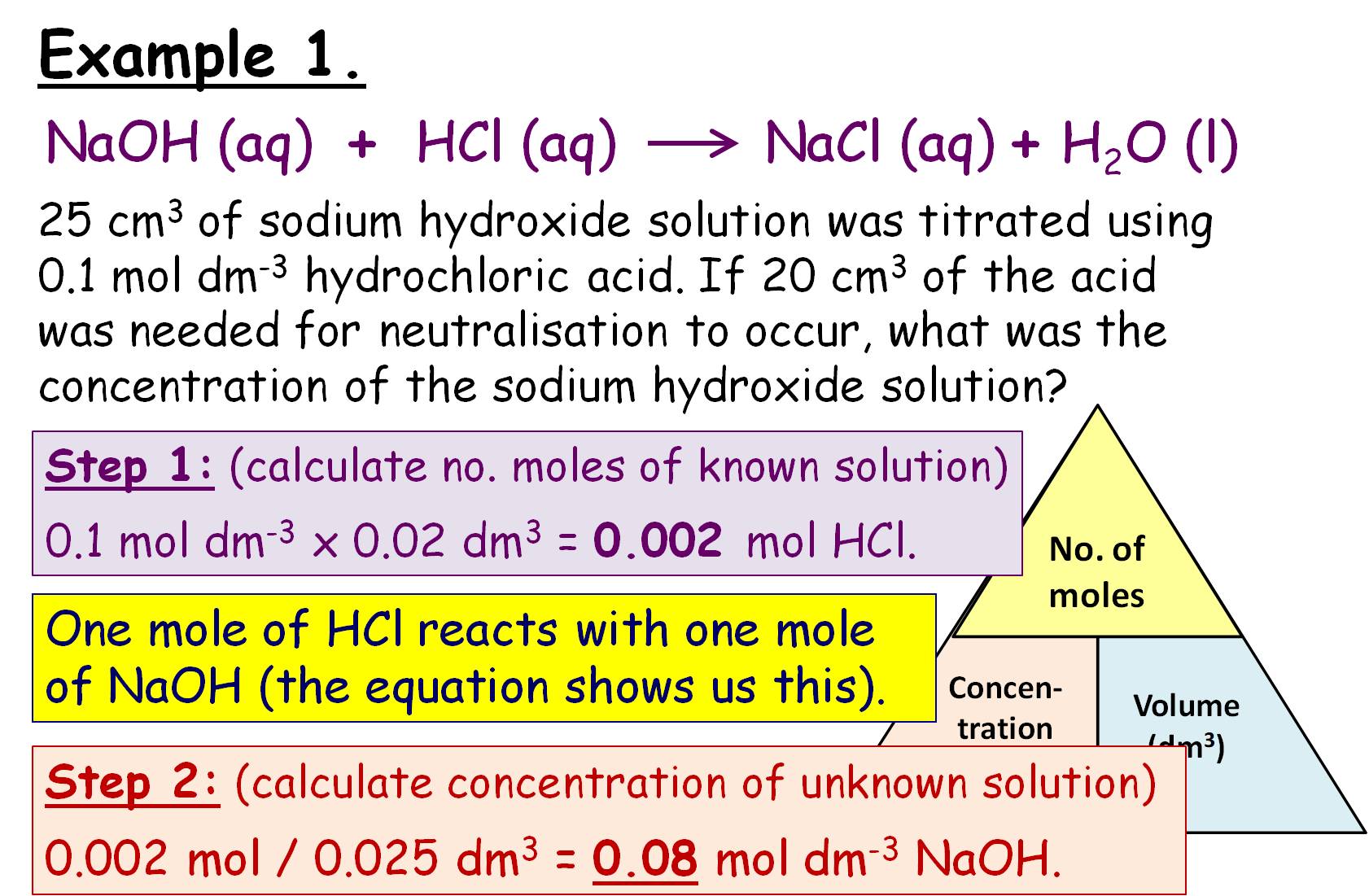
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d
Titration Basic Equation Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Calculate the ph at these. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculate the amount of sodium hydroxide in moles. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Basic Equation A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Volume of sodium hydroxide solution =. Titration Basic Equation.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration Basic Equation Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. The above equation works only for neutralizations. Titration Basic Equation.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Basic Equation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration. Titration Basic Equation.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Basic Equation Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Volume of sodium hydroxide. Titration Basic Equation.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The above equation works only for. Titration Basic Equation.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Basic Equation Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Volume of sodium hydroxide solution =. Titration Basic Equation.
From www.youtube.com
Titration Calculations YouTube Titration Basic Equation Calculate the amount of sodium hydroxide in moles. Calculate the ph at these. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Basic Equation.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration Basic Equation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. Titration, also known as titrimetry,. Titration Basic Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Basic Equation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Calculate the ph at these. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculate the amount of sodium hydroxide in moles.. Titration Basic Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Basic Equation Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculate the ph at these. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another. Titration Basic Equation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Basic Equation Calculate the ph at these. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a. Titration Basic Equation.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Basic Equation A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A titration is. Titration Basic Equation.
From mungfali.com
Acid Base Titration Method Titration Basic Equation A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one. Titration Basic Equation.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration Basic Equation A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Calculate the amount of sodium hydroxide in moles. Calculate the ph at these. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution. Titration Basic Equation.
From mungfali.com
HCl NaOH Titration Titration Basic Equation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a. Titration Basic Equation.
From mavink.com
Titration Labeled Titration Basic Equation Calculate the amount of sodium hydroxide in moles. Calculate the ph at these. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100. Titration Basic Equation.
From ar.inspiredpencil.com
Titration Equation Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Titration Basic Equation.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is a volumetric technique in which a solution of one. Titration Basic Equation.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Basic Equation Calculate the ph at these. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Calculate the amount of sodium hydroxide in moles. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100. Titration Basic Equation.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Calculate the amount of sodium hydroxide in moles. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration. Titration Basic Equation.
From ar.inspiredpencil.com
Titration Equation Titration Basic Equation A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Basic Equation.
From www.dreamstime.com
Titration Stock Illustrations 259 Titration Stock Illustrations Titration Basic Equation A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Calculate the amount of sodium hydroxide in moles. Titration, also known as titrimetry, is a chemical. Titration Basic Equation.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Basic Equation A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is carried out for 25.00 ml of 0.100 m hcl. Titration Basic Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is a. Titration Basic Equation.
From www.savemyexams.com
Prepare a Salt by Titration Edexcel GCSE Chemistry Revision Notes 2018 Titration Basic Equation Calculate the ph at these. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Calculate the amount of sodium hydroxide in moles. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume. Titration Basic Equation.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Basic Equation Calculate the amount of sodium hydroxide in moles. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration Basic Equation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Basic Equation The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual. Titration Basic Equation.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Basic Equation Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Titration involves the gradual addition of. Titration Basic Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Calculate the ph at these. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Basic Equation.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Basic Equation A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration. Titration Basic Equation.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Basic Equation Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 =. Titration Basic Equation.
From ar.inspiredpencil.com
Titration Equation Titration Basic Equation Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Calculate the ph at. Titration Basic Equation.
From mavink.com
Acid Base Titration Equation Titration Basic Equation A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Calculate the ph at these. A. Titration Basic Equation.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Basic Equation Calculate the amount of sodium hydroxide in moles. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is an experiment in which a solution, whose concentration is known, is gradually added to a measured volume of another solution in order to determine its. A titration is a. Titration Basic Equation.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Basic Equation The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is an experiment in which a solution, whose concentration is known, is gradually. Titration Basic Equation.