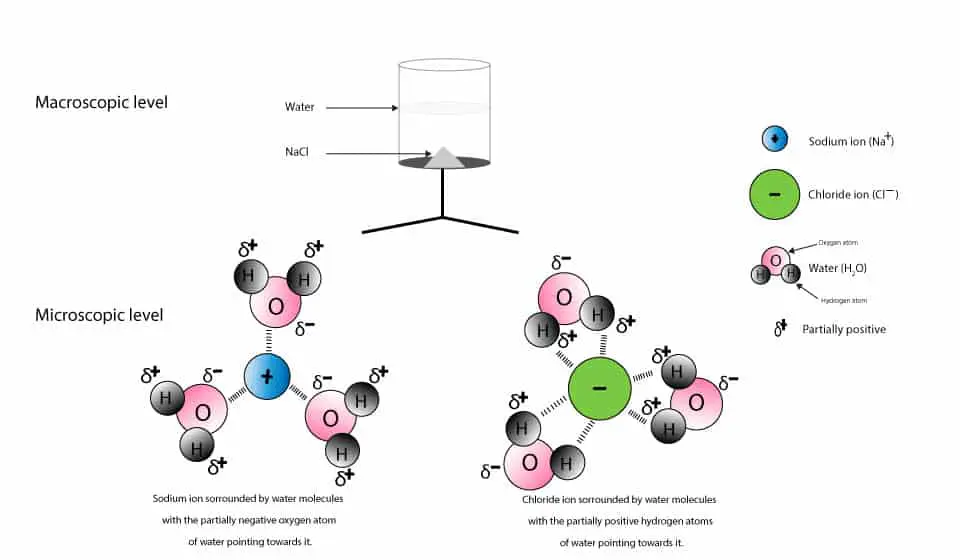
Table Salt Chemical Formula Single Displacement . There are three basic ways you will need to know that are used to describe aqueous chemical reactions. The difference between these lies in how you describe the ions of. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. In these kinds of reactions one. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The chemical formula of sodium chloride is nacl. These are typically single and double displacement reactions. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). Single displacement (substitution) when one element trades places with another element in a compound. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. A single displacement reaction occurs when another element in a compound is replaced by an element.
from awesomehome.co
The chemical formula of sodium chloride is nacl. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. In these kinds of reactions one. These are typically single and double displacement reactions. The difference between these lies in how you describe the ions of. A single displacement reaction occurs when another element in a compound is replaced by an element.
Table Salt Chemical Formula Equation Awesome Home
Table Salt Chemical Formula Single Displacement The difference between these lies in how you describe the ions of. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. Single displacement (substitution) when one element trades places with another element in a compound. A single displacement reaction occurs when another element in a compound is replaced by an element. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. These are typically single and double displacement reactions. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. The chemical formula of sodium chloride is nacl. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). In these kinds of reactions one. The difference between these lies in how you describe the ions of. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Chemical Formula Single Displacement There are three basic ways you will need to know that are used to describe aqueous chemical reactions. The difference between these lies in how you describe the ions of. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a).. Table Salt Chemical Formula Single Displacement.
From ar.inspiredpencil.com
Table Salt Molecular Structure Table Salt Chemical Formula Single Displacement In these kinds of reactions one. The chemical formula of sodium chloride is nacl. These are typically single and double displacement reactions. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. A single displacement reaction occurs when another element in a compound is replaced by an element.. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
What Is The Chemical Formula For Table Salt Elcho Table Table Salt Chemical Formula Single Displacement The positive and negative regions on the water molecule (the hydrogen and oxygen ends. These are typically single and double displacement reactions. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The chemical formula of sodium chloride is nacl. Carry out single displacement reactions involving (a) metals. Table Salt Chemical Formula Single Displacement.
From www.slideserve.com
PPT Stability and Ionic Bonding PowerPoint Presentation, free Table Salt Chemical Formula Single Displacement There are three basic ways you will need to know that are used to describe aqueous chemical reactions. These are typically single and double displacement reactions. A single displacement reaction occurs when another element in a compound is replaced by an element. Single displacement (substitution) when one element trades places with another element in a compound. A metal only substitutes. Table Salt Chemical Formula Single Displacement.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Table Salt Chemical Formula Single Displacement There are three basic ways you will need to know that are used to describe aqueous chemical reactions. In these kinds of reactions one. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. These are typically single and double displacement reactions. This formula indicates that each unit of sodium chloride is composed of one. Table Salt Chemical Formula Single Displacement.
From www.animalia-life.club
Table Salt Chemical Structure Table Salt Chemical Formula Single Displacement Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. A. Table Salt Chemical Formula Single Displacement.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Chemical Formula Single Displacement Single displacement (substitution) when one element trades places with another element in a compound. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. In these kinds of reactions one. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). There are three basic ways you will need to know that. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Equation For Table Salt Elcho Table Table Salt Chemical Formula Single Displacement A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. The difference between these lies in how you describe the ions of. In these kinds of reactions one. The chemical formula of sodium chloride is nacl. A single displacement reaction occurs when another element in a compound is replaced by an element. Carry out single. Table Salt Chemical Formula Single Displacement.
From www.animalia-life.club
Table Salt Chemical Structure Table Salt Chemical Formula Single Displacement The difference between these lies in how you describe the ions of. These are typically single and double displacement reactions. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. A single displacement. Table Salt Chemical Formula Single Displacement.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula Table Salt Chemical Formula Single Displacement A single displacement reaction occurs when another element in a compound is replaced by an element. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. The difference between these lies in how you describe the ions of. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one. Table Salt Chemical Formula Single Displacement.
From giopcwnln.blob.core.windows.net
Table Salt Chemical at Susan Bolinger blog Table Salt Chemical Formula Single Displacement The difference between these lies in how you describe the ions of. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. The chemical formula of sodium chloride is nacl. The positive and negative regions on the water molecule (the. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Formula For Common Table Salt Elcho Table Table Salt Chemical Formula Single Displacement A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. The difference between these lies in how you describe the ions of. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one. Table Salt Chemical Formula Single Displacement.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Chemical Formula Single Displacement A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). In these kinds of reactions one. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. The difference between these lies in how you describe the ions of. A single displacement reaction occurs when another element. Table Salt Chemical Formula Single Displacement.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Chemical Formula Single Displacement The difference between these lies in how you describe the ions of. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). The positive and negative regions on the water molecule (the hydrogen and oxygen ends. A single displacement reaction occurs when another element in a compound is replaced by an element. Single displacement (substitution). Table Salt Chemical Formula Single Displacement.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Chemical Formula Single Displacement Single displacement (substitution) when one element trades places with another element in a compound. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. This formula indicates that each unit of sodium chloride is composed of one. Table Salt Chemical Formula Single Displacement.
From riversfansite.blogspot.com
Table Salt Chemical Formula Sodium chloride , commonly known as salt Table Salt Chemical Formula Single Displacement The difference between these lies in how you describe the ions of. A single displacement reaction occurs when another element in a compound is replaced by an element. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Single displacement (substitution) when one element trades places with another. Table Salt Chemical Formula Single Displacement.
From exocimcrd.blob.core.windows.net
Table Salt Chemical Structure at Deangelo Clark blog Table Salt Chemical Formula Single Displacement This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Single displacement (substitution) when one element trades places with another element in a compound. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. Carry out single displacement reactions involving (a) metals with. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Name For Table Salt Elcho Table Table Salt Chemical Formula Single Displacement A single displacement reaction occurs when another element in a compound is replaced by an element. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. A common salt, nacl, also known as table salt, dissociates. Table Salt Chemical Formula Single Displacement.
From exomdamui.blob.core.windows.net
Table Salt Atomic Formula at Pete Alvarez blog Table Salt Chemical Formula Single Displacement These are typically single and double displacement reactions. The difference between these lies in how you describe the ions of. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. A single displacement reaction occurs when another element in a compound is replaced by an element. The positive. Table Salt Chemical Formula Single Displacement.
From www.thoughtco.com
Table Salt Molecular Formula Sodium Chloride Table Salt Chemical Formula Single Displacement The chemical formula of sodium chloride is nacl. Single displacement (substitution) when one element trades places with another element in a compound. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. These are typically single and double displacement reactions. Carry out single displacement reactions involving (a) metals with salts of other. Table Salt Chemical Formula Single Displacement.
From www.alamy.com
Sodium chloride (table salt), chemical structure. Blue skeletal formula Table Salt Chemical Formula Single Displacement The positive and negative regions on the water molecule (the hydrogen and oxygen ends. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. The chemical formula of sodium chloride. Table Salt Chemical Formula Single Displacement.
From riversfansite.blogspot.com
Table Salt Chemical Formula Sodium chloride , commonly known as salt Table Salt Chemical Formula Single Displacement The positive and negative regions on the water molecule (the hydrogen and oxygen ends. These are typically single and double displacement reactions. Single displacement (substitution) when one element trades places with another element in a compound. The chemical formula of sodium chloride is nacl. The difference between these lies in how you describe the ions of. A common salt, nacl,. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Table Salt Chemical Formula Single Displacement Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. A. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Equation For Table Salt Elcho Table Table Salt Chemical Formula Single Displacement This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. These are typically single and double displacement reactions. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. A. Table Salt Chemical Formula Single Displacement.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Table Salt Chemical Formula Single Displacement The chemical formula of sodium chloride is nacl. Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. A single displacement reaction occurs when another element in a compound is replaced by an element. Single displacement (substitution) when one element trades places with another element in a compound. The positive and. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Formula For Table Salt Elcho Table Table Salt Chemical Formula Single Displacement In these kinds of reactions one. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). Single displacement (substitution) when one element trades places with another element in a compound. The chemical formula of sodium chloride is nacl. The positive. Table Salt Chemical Formula Single Displacement.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Chemical Formula Single Displacement This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). The difference between these lies in how you describe the ions of. In these kinds of reactions one. Single displacement (substitution) when one. Table Salt Chemical Formula Single Displacement.
From slideplayer.com
Chapter 5 Objectives Distinguish between compounds and mixtures. ppt Table Salt Chemical Formula Single Displacement There are three basic ways you will need to know that are used to describe aqueous chemical reactions. The difference between these lies in how you describe the ions of. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal.. Table Salt Chemical Formula Single Displacement.
From elchoroukhost.net
Chemical Formula For Table Salt Elcho Table Table Salt Chemical Formula Single Displacement The chemical formula of sodium chloride is nacl. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). The difference between these lies in how you describe the ions of. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. There are three basic ways you will need to know. Table Salt Chemical Formula Single Displacement.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Table Salt Chemical Formula Single Displacement There are three basic ways you will need to know that are used to describe aqueous chemical reactions. In these kinds of reactions one. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The difference between these lies in how you describe the ions of. A single. Table Salt Chemical Formula Single Displacement.
From eduinput.com
30 Examples Of Common Chemical Compounds with Chemical Formula Table Salt Chemical Formula Single Displacement A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). The difference between these lies in how you describe the ions of. A single displacement reaction occurs when another element in a compound is replaced by an element. The chemical formula of sodium chloride is nacl. There are three basic ways you will need to. Table Salt Chemical Formula Single Displacement.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Table Salt Chemical Formula Single Displacement This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. A single displacement reaction occurs when another element in a compound is replaced by an element. The positive and negative regions on the water molecule (the hydrogen and oxygen ends. The chemical formula of sodium chloride is nacl.. Table Salt Chemical Formula Single Displacement.
From awesomehome.co
Table Salt Chemical Formula Awesome Home Table Salt Chemical Formula Single Displacement Carry out single displacement reactions involving (a) metals with salts of other metals, and (b) metals with dilute acid. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Single displacement (substitution) when one element trades places with another element in a compound. The positive and negative regions. Table Salt Chemical Formula Single Displacement.
From www.animalia-life.club
Table Salt Chemical Structure Table Salt Chemical Formula Single Displacement There are three basic ways you will need to know that are used to describe aqueous chemical reactions. A single displacement reaction occurs when another element in a compound is replaced by an element. A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). This formula indicates that each unit of sodium chloride is composed. Table Salt Chemical Formula Single Displacement.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Chemical Formula Single Displacement A common salt, nacl, also known as table salt, dissociates completely in water (figure 15.1a). Single displacement (substitution) when one element trades places with another element in a compound. There are three basic ways you will need to know that are used to describe aqueous chemical reactions. This formula indicates that each unit of sodium chloride is composed of one. Table Salt Chemical Formula Single Displacement.