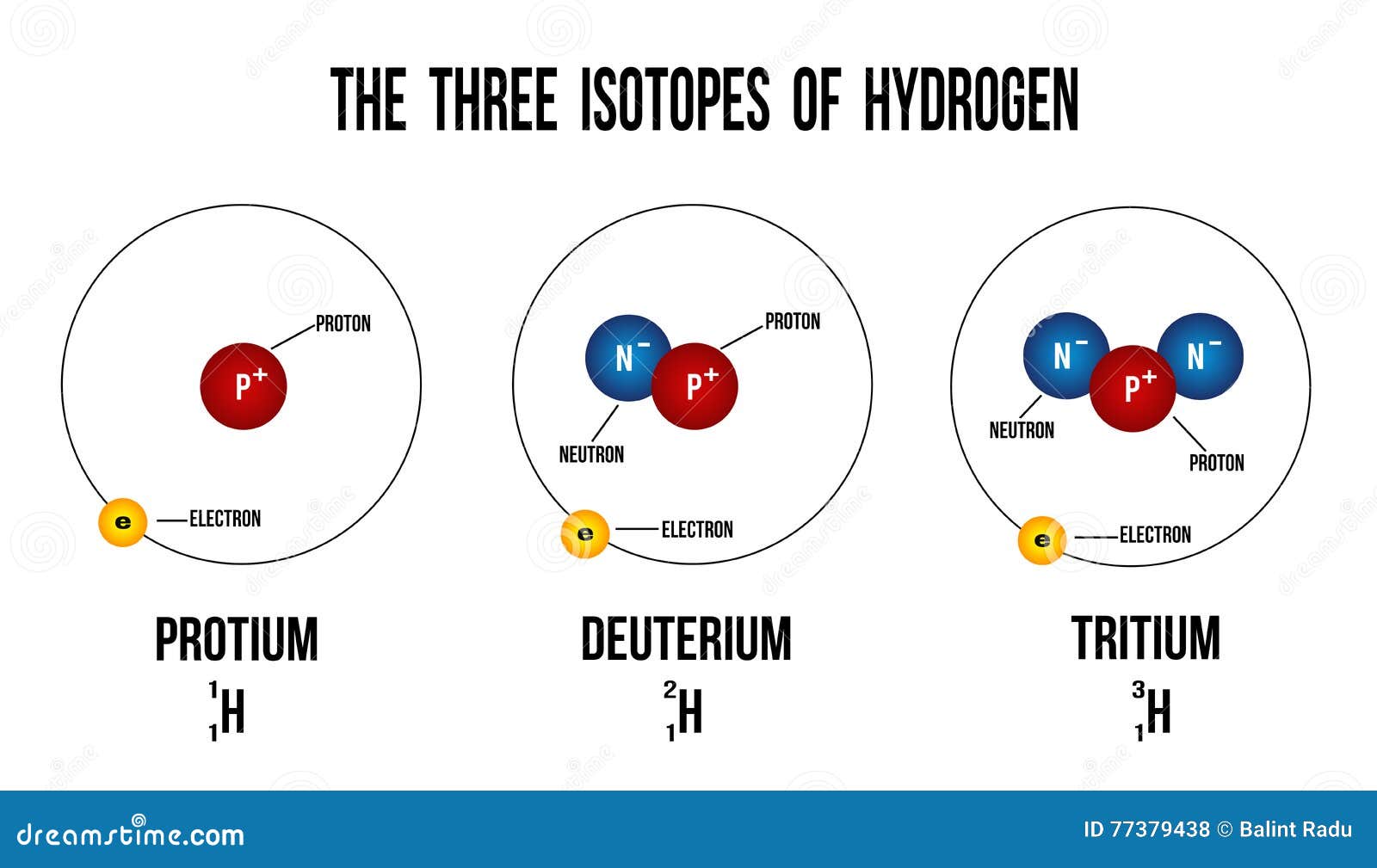
How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other . Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. They differ in the number of neutrons in their. The three isotopes of hydrogen are protium, deuterium, and tritium. There are three isotopes of the element hydrogen: Their atomic masses are different. They each have one single proton (z = 1), but differ in the. How do we distinguish between them? Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h.
from www.dreamstime.com
How do we distinguish between them? They differ in the number of neutrons in their. The three isotopes of hydrogen are protium, deuterium, and tritium. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. There are three isotopes of the element hydrogen: They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Their atomic masses are different.
The Three Isotopes of Hydrogen Stock Vector Illustration of isotope
How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. There are three isotopes of the element hydrogen: How do we distinguish between them? They differ in the number of neutrons in their. Their atomic masses are different.
From www.slideserve.com
PPT Bohr’s model of H atom PowerPoint Presentation ID169224 How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They differ in the number of neutrons in their. The three isotopes of hydrogen are protium, deuterium, and tritium. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. How do we distinguish between them? Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Their atomic masses are different. There are. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.vectorstock.com
H2 hydrogen molecule Royalty Free Vector Image How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other How do we distinguish between them? They each have one single proton (z = 1), but differ in the. They differ in the number of neutrons in their. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. There are three isotopes. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.youtube.com
Hydrogen Electron Configuration YouTube How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Their atomic masses are different. They differ in the number of neutrons in their. How do we distinguish between them? Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From valenceelectrons.com
Hydrogen Electron Configuration And Full Orbital Diagram How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. Their atomic masses are different. There are three isotopes of the element hydrogen: Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. How do we distinguish between them? The three isotopes of hydrogen are protium, deuterium, and tritium. Other, highly unstable nuclei. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.dreamstime.com
Hydrogen Isotopes. Atomic Structure Stock Vector Illustration of How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other There are three isotopes of the element hydrogen: Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ in the. How do we distinguish between them? Other, highly unstable nuclei (4 h to 7 h). How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.vectorstock.com
Diagram representation element hydrogen Royalty Free Vector How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. How do we distinguish between them? The three isotopes of hydrogen are protium, deuterium, and tritium. There are three isotopes of the element hydrogen: Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Their atomic masses are different. They differ in the. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.britannica.com
Hydrogen Properties, Uses, & Facts Britannica How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other There are three isotopes of the element hydrogen: The three isotopes of hydrogen are protium, deuterium, and tritium. They differ in the number of neutrons in their. Their atomic masses are different. How do we distinguish between them? Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. They each have one single proton (z =. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From mavink.com
Periodic Table Hydrogen Facts How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They differ in the number of neutrons in their. Their atomic masses are different. They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. There are three isotopes of. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From animalia-life.club
Bohr Atomic Model Of Hydrogen How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other The three isotopes of hydrogen are protium, deuterium, and tritium. Their atomic masses are different. They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. They differ in the. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From pdfslide.net
(PPT) Review Draw a model of each of the hydrogen isotopes Hydrogen1 How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. The three isotopes of hydrogen are protium, deuterium, and tritium. They differ in the number of neutrons in their. How do we distinguish between them? There are three isotopes of the element. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From beechsciencerhymery.blogspot.com
Science Mnemomic Rhymes Chemistry How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They differ in the number of neutrons in their. They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Their atomic masses are different. The three isotopes of hydrogen are protium, deuterium, and tritium. How do we distinguish between them? Other, highly unstable. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.doubtnut.com
How is hydrogen molecule formed How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. They differ in the number of neutrons in their. There are three isotopes of the element hydrogen: The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.alamy.com
Hydrogen atom orbital structure hires stock photography and images Alamy How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other How do we distinguish between them? The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ in the. They differ in the number of neutrons in their. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Hydrogen has three naturally occurring isotopes, denoted 1. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From mavink.com
Periodic Table Hydrogen Facts How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. They differ in the number of neutrons in their. How do we distinguish between them? Their atomic masses are different. There are three isotopes of the element hydrogen: The three isotopes of hydrogen. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.aquaportail.com
Hydrogène définition et explications How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. How do we distinguish between them? They each have one single proton (z = 1), but differ in the. They differ in the number of neutrons in their. There are three isotopes of the element hydrogen: The three isotopes of hydrogen are protium, deuterium, and tritium.. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.sliderbase.com
Properties of Water Presentation Biology How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. They differ in the number of neutrons in their. They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. The three isotopes of hydrogen are protium, deuterium, and tritium.. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From mavink.com
Hydrogen Periodic Table Neutrons How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other How do we distinguish between them? The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ in the. There are three isotopes of the element hydrogen: They differ in the number of neutrons in their. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h.. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. They each have one single proton (z = 1), but differ in the. Their atomic masses are different. How do we distinguish between them? Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. There are three isotopes of the element hydrogen:. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.youtube.com
ISOTOPES OF HYDROGEN HYDROGEN 3 YouTube How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. How do we distinguish between them? There are three isotopes of the element hydrogen: They differ in the number of neutrons in their. Their atomic masses are different. They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.alamy.com
Hydrogen atom diagram concept Stock Vector Image & Art Alamy How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. Their atomic masses are different. The three isotopes of hydrogen are protium, deuterium, and tritium. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. How do we distinguish between them? They differ in the number of neutrons in their. Other, highly unstable. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From sciencenotes.org
Types of Chemical Bonds How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Their atomic masses are different. They differ in the number of neutrons in their. How do we distinguish between them? Other, highly unstable. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.vectorstock.com
Hydrogen atom on white background Royalty Free Vector Image How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Their atomic masses are different. There are three isotopes of the element hydrogen: How do we distinguish between them? They each have one single proton (z = 1), but differ in the. The three isotopes of hydrogen are protium, deuterium, and tritium. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Hydrogen has three naturally. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From valenceelectrons.com
Electron Configuration for Hydrogen Full Explanation How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Their atomic masses are different. How do we distinguish between them? Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.dreamstime.com
The Three Isotopes of Hydrogen Stock Vector Illustration of isotope How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. The three isotopes of hydrogen are protium, deuterium, and tritium. Their atomic masses are different. How do we distinguish between them? They differ in the number of neutrons in their. There are three isotopes of the element hydrogen: Hydrogen has three naturally occurring isotopes, denoted 1 h,. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other The three isotopes of hydrogen are protium, deuterium, and tritium. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. They each have one single proton (z = 1), but differ in the. They differ in the number of neutrons in their. How do we distinguish between them? There are three isotopes of the element hydrogen:. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From mavink.com
Electron Configuration Of Hydrogen How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Their atomic masses are different. They differ in the number of neutrons in their. The three isotopes of hydrogen are protium, deuterium, and tritium. There are three isotopes of the element hydrogen: They each have one single proton (z = 1), but differ in the. How do we distinguish between them? Hydrogen has three naturally occurring isotopes, denoted 1 h,. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.numerade.com
SOLVED 70 ompleted 18 out of 20 How does 'H (hydrogen3) differ from How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other The three isotopes of hydrogen are protium, deuterium, and tritium. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. There are three isotopes of the element hydrogen: They each have one single proton (z = 1), but differ in the. Their. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.wikihow.com
How to Learn About the Chemistry of the Hydrogen Atom 12 Steps How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. How do we distinguish between them? Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Their atomic masses are different. The three isotopes of hydrogen are protium, deuterium,. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.animalia-life.club
Electron Configuration Of Hydrogen How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. Their atomic masses are different. How do we distinguish between them? There are three isotopes of the element hydrogen: Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. The three isotopes of hydrogen are protium, deuterium, and tritium. They each have. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.dreamstime.com
Element of Hydrogen stock vector. Illustration of university 89525839 How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other How do we distinguish between them? Their atomic masses are different. They differ in the number of neutrons in their. Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. The three isotopes of hydrogen are protium, deuterium, and tritium. There are three isotopes of the element hydrogen: They each have one single proton (z =. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.thoughtco.com
First 20 Elements of the Periodic Table How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other The three isotopes of hydrogen are protium, deuterium, and tritium. They each have one single proton (z = 1), but differ in the. They differ in the number of neutrons in their. Their atomic masses are different. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. There are three isotopes of the element hydrogen: Other,. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other Other, highly unstable nuclei (4 h to 7 h) have been synthesized in the. They each have one single proton (z = 1), but differ in the. The three isotopes of hydrogen are protium, deuterium, and tritium. How do we distinguish between them? Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Their atomic masses. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From newtondesk.com
Hydrogen Element (Periodic Table) Reactions, Properties, & Uses Chem How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other How do we distinguish between them? The three isotopes of hydrogen are protium, deuterium, and tritium. There are three isotopes of the element hydrogen: They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Other, highly unstable nuclei (4 h to 7 h). How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From sklep.foteks.pl
Plakat hydrogen bond. chemistry lesson. Infographic. hydrogen bonds How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They differ in the number of neutrons in their. The three isotopes of hydrogen are protium, deuterium, and tritium. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. Their atomic masses are different. How do we distinguish between them? They each have one single proton (z = 1), but differ in the. Other, highly unstable. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.
From www.biologyonline.com
Hydrogen Definition and Examples Biology Online Dictionary How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other They each have one single proton (z = 1), but differ in the. Hydrogen has three naturally occurring isotopes, denoted 1 h, 2 h and 3 h. There are three isotopes of the element hydrogen: They differ in the number of neutrons in their. Their atomic masses are different. The three isotopes of hydrogen are protium, deuterium, and tritium. Other,. How Do Hydrogen-1 Hydrogen-2 And Hydrogen-3 Differ From Each Other.