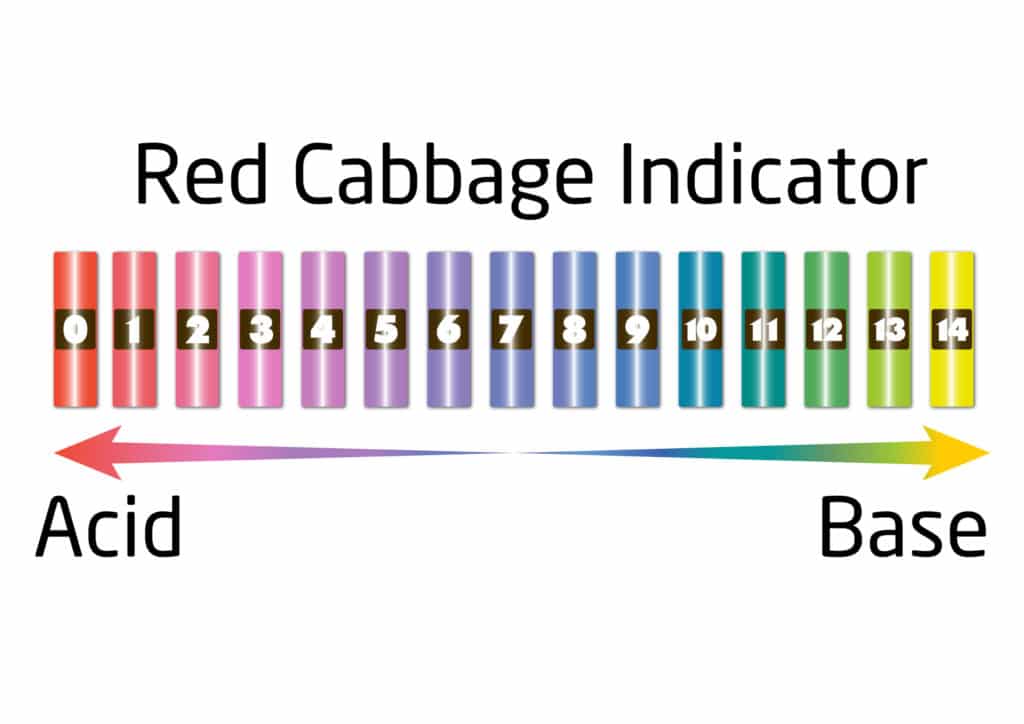
An Indicator For Detecting An Acid Or Base . They are commonly used in chemistry to visually. This is a charge of common. An indicator is a weak acid that ionizes. Substances such as phenolphthalein, which can be. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Only a small amount of indicator compound is needed to produce a visible color change. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This page assumes that you know.
from www.science-sparks.com
Substances such as phenolphthalein, which can be. This page assumes that you know. An indicator is a weak acid that ionizes. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. Only a small amount of indicator compound is needed to produce a visible color change. They are commonly used in chemistry to visually. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. This is a charge of common.
How to make a red cabbage pH indicator Chemistry for Kids
An Indicator For Detecting An Acid Or Base This is a charge of common. Only a small amount of indicator compound is needed to produce a visible color change. This is a charge of common. Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes. This page assumes that you know. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. They are commonly used in chemistry to visually.
From www.science-sparks.com
How to make a red cabbage pH indicator Chemistry for Kids An Indicator For Detecting An Acid Or Base Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph. An Indicator For Detecting An Acid Or Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo An Indicator For Detecting An Acid Or Base An indicator is a weak acid that ionizes. This is a charge of common. Substances such as phenolphthalein, which can be. Only a small amount of indicator compound is needed to produce a visible color change. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. An Indicator For Detecting An Acid Or Base.
From www.youtube.com
What is an Indicator? What Are AcidBase Indicators Class 10 Science An Indicator For Detecting An Acid Or Base This is a charge of common. Only a small amount of indicator compound is needed to produce a visible color change. They are commonly used in chemistry to visually. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. An indicator is a weak acid. An Indicator For Detecting An Acid Or Base.
From www.youtube.com
ACID BASE INDICATORS YouTube An Indicator For Detecting An Acid Or Base An indicator is a weak acid that ionizes. This is a charge of common. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l. An Indicator For Detecting An Acid Or Base.
From www.carolina.com
Acid Universal Indicator, pH 17, Laboratory Chemical Grade Carolina An Indicator For Detecting An Acid Or Base This is a charge of common. An indicator is a weak acid that ionizes. Only a small amount of indicator compound is needed to produce a visible color change. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. In more basic solutions where the hydronium. An Indicator For Detecting An Acid Or Base.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 An Indicator For Detecting An Acid Or Base Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. An indicator is a weak acid that ionizes. They are commonly used in chemistry to visually. In more. An Indicator For Detecting An Acid Or Base.
From www.chemedx.org
Making Natural Acid Base Indicators Chemical Education Xchange An Indicator For Detecting An Acid Or Base Only a small amount of indicator compound is needed to produce a visible color change. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. This page assumes that you know. This is a charge of common. Substances such as phenolphthalein, which can be. In more. An Indicator For Detecting An Acid Or Base.
From crossword-solver.io
Acid or base indicator Crossword Clue Answers Crossword Solver An Indicator For Detecting An Acid Or Base This page assumes that you know. An indicator is a weak acid that ionizes. They are commonly used in chemistry to visually. This is a charge of common. Substances such as phenolphthalein, which can be. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) →. An Indicator For Detecting An Acid Or Base.
From brainly.in
what is an acidbase indicator? give one example. Brainly.in An Indicator For Detecting An Acid Or Base Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes. Only a small. An Indicator For Detecting An Acid Or Base.
From gioolhlbr.blob.core.windows.net
An Acid/Base Indicator Example at Donna Kim blog An Indicator For Detecting An Acid Or Base Only a small amount of indicator compound is needed to produce a visible color change. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Substances such as. An Indicator For Detecting An Acid Or Base.
From www.teachoo.com
Turmeric as an Acid Base Indicator How is it used? (with Examples) An Indicator For Detecting An Acid Or Base In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can be. This is a charge of common. They are commonly used in chemistry to visually. This page assumes that you know. Only a small amount of indicator compound is. An Indicator For Detecting An Acid Or Base.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ An Indicator For Detecting An Acid Or Base An indicator is a weak acid that ionizes. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. In more basic solutions where the hydronium ion concentration is. An Indicator For Detecting An Acid Or Base.
From www.youtube.com
Acid Base Indicators Introduction Acids and Bases Chemistry Practice An Indicator For Detecting An Acid Or Base This page assumes that you know. This is a charge of common. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. They are commonly used in chemistry to visually. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3. An Indicator For Detecting An Acid Or Base.
From knowledge.carolina.com
AcidBase Indicators Carolina Knowledge Center An Indicator For Detecting An Acid Or Base This page assumes that you know. They are commonly used in chemistry to visually. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is. An Indicator For Detecting An Acid Or Base.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts An Indicator For Detecting An Acid Or Base This is a charge of common. An indicator is a weak acid that ionizes. Substances such as phenolphthalein, which can be. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. They are commonly used in chemistry to visually. This page assumes that you know.. An Indicator For Detecting An Acid Or Base.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations An Indicator For Detecting An Acid Or Base Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Substances such as phenolphthalein, which can be. This page assumes that you know. They are commonly used in. An Indicator For Detecting An Acid Or Base.
From homeschoolden.com
Experiment 13 Red Cabbage pH Indicator, AcidBase Tests Homeschool Den An Indicator For Detecting An Acid Or Base In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This page assumes that you know. Substances such as phenolphthalein, which can be. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l. An Indicator For Detecting An Acid Or Base.
From www.chemistry4students.com
Chemistry 4 Students Common AcidBase Indicators An Indicator For Detecting An Acid Or Base Substances such as phenolphthalein, which can be. Only a small amount of indicator compound is needed to produce a visible color change. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. This page assumes that you know. They are commonly used in chemistry to visually.. An Indicator For Detecting An Acid Or Base.
From www.thoughtco.com
Definition and Examples of AcidBase Indicator An Indicator For Detecting An Acid Or Base Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Only a small. An Indicator For Detecting An Acid Or Base.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID An Indicator For Detecting An Acid Or Base They are commonly used in chemistry to visually. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. An indicator is a weak acid that ionizes. This is. An Indicator For Detecting An Acid Or Base.
From slideplayer.com
Acid, Base, or Neutral. ppt download An Indicator For Detecting An Acid Or Base When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This page assumes that you know. An indicator is a weak. An Indicator For Detecting An Acid Or Base.
From www.chegg.com
Solved Choose the correct indicator for acidbase titration An Indicator For Detecting An Acid Or Base Only a small amount of indicator compound is needed to produce a visible color change. This is a charge of common. Substances such as phenolphthalein, which can be. This page assumes that you know. They are commonly used in chemistry to visually. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity. An Indicator For Detecting An Acid Or Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 An Indicator For Detecting An Acid Or Base Only a small amount of indicator compound is needed to produce a visible color change. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. An indicator is a weak acid that ionizes. Substances such as phenolphthalein, which can be. They are commonly used in. An Indicator For Detecting An Acid Or Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo An Indicator For Detecting An Acid Or Base Only a small amount of indicator compound is needed to produce a visible color change. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This is a charge of common. Substances such as phenolphthalein, which can be. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g). An Indicator For Detecting An Acid Or Base.
From www.youtube.com
R3.1.14 / R3.1.15 Acidbase indicators (HL) YouTube An Indicator For Detecting An Acid Or Base Only a small amount of indicator compound is needed to produce a visible color change. This page assumes that you know. Substances such as phenolphthalein, which can be. They are commonly used in chemistry to visually. An indicator is a weak acid that ionizes. When used as a dilute solution, a ph indicator does not have a significant impact on. An Indicator For Detecting An Acid Or Base.
From www.youtube.com
Acid or Base? Use an Indicator! YouTube An Indicator For Detecting An Acid Or Base This is a charge of common. They are commonly used in chemistry to visually. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Only a small amount. An Indicator For Detecting An Acid Or Base.
From slideplayer.com
Unit 14 Acid, Bases, & Salts ppt download An Indicator For Detecting An Acid Or Base Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Only a small amount of indicator compound is needed to produce a visible color change. When used as. An Indicator For Detecting An Acid Or Base.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry An Indicator For Detecting An Acid Or Base When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. This is a charge of common. This page assumes that you know. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. An Indicator For Detecting An Acid Or Base.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector An Indicator For Detecting An Acid Or Base They are commonly used in chemistry to visually. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. An indicator is a weak acid that ionizes. Only a small amount of indicator compound is needed to produce a visible color change. Nahco3(aq) + hcl(aq) →. An Indicator For Detecting An Acid Or Base.
From slideplayer.com
The pH Scale Is a substance an acid or a base?. ACIDS An acid is a An Indicator For Detecting An Acid Or Base In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Only a small amount of indicator compound is needed to produce a visible color change. They are commonly used in chemistry to visually. An indicator is a weak acid that ionizes. This is a charge. An Indicator For Detecting An Acid Or Base.
From pediaa.com
Difference Between Acid Base Indicator and Universal Indicator An Indicator For Detecting An Acid Or Base An indicator is a weak acid that ionizes. Substances such as phenolphthalein, which can be. When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. This is a charge of common. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o. An Indicator For Detecting An Acid Or Base.
From www.teachoo.com
[MCQ] Which one of the following can be used as an acidbase indicator An Indicator For Detecting An Acid Or Base They are commonly used in chemistry to visually. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. Substances such as phenolphthalein, which can be. An indicator is. An Indicator For Detecting An Acid Or Base.
From www.slideserve.com
PPT Litmus Indicator PowerPoint Presentation ID2790323 An Indicator For Detecting An Acid Or Base Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l (a q) → n a c l (a q) + c o 2 (g) + h 2 o (l) 6. An indicator is a weak acid that ionizes. They are commonly used in chemistry to visually. This is. An Indicator For Detecting An Acid Or Base.
From www.chegg.com
Solved 50 Determination of the Molarity of an Acid or Base An Indicator For Detecting An Acid Or Base In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This is a charge of common. Substances such as phenolphthalein, which can be. Nahco3(aq) + hcl(aq) → nacl(aq) + co2(g) +h2o(l) (1.6.2) (1.6.2) n a h c o 3 (a q) + h c l. An Indicator For Detecting An Acid Or Base.
From study.com
AcidBase Indicators Uses & Examples Video & Lesson Transcript An Indicator For Detecting An Acid Or Base When used as a dilute solution, a ph indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can be. This page assumes that. An Indicator For Detecting An Acid Or Base.