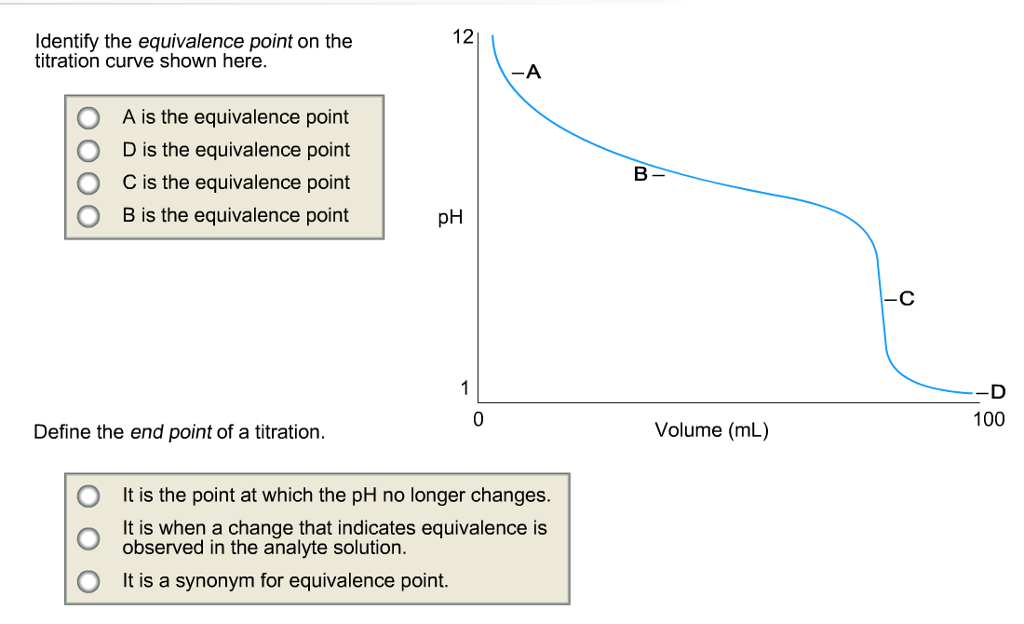
Equivalence Point Of The Titration Curve . Sorting out some confusing terms. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Nearer to the equivalence point,. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. the equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. As base is added to acid at the beginning of a titration, the ph rises very slowly. equivalence point (v = 25 ml): a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.
from www.chegg.com
the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. the equivalence point of a titration. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Nearer to the equivalence point,. As base is added to acid at the beginning of a titration, the ph rises very slowly. equivalence point (v = 25 ml): Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid.
Solved Identify the equivalence point on the titration curve
Equivalence Point Of The Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. equivalence point (v = 25 ml): As base is added to acid at the beginning of a titration, the ph rises very slowly. Nearer to the equivalence point,. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Sorting out some confusing terms. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the equivalence point of a titration.
From giorjuuhy.blob.core.windows.net
Titration Curve With Points at Geneva Sampson blog Equivalence Point Of The Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. the equivalence point of a titration. a typical titration curve of a diprotic acid, oxalic. Equivalence Point Of The Titration Curve.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Equivalence Point Of The Titration Curve As base is added to acid at the beginning of a titration, the ph rises very slowly. the equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong. Equivalence Point Of The Titration Curve.
From www.vrogue.co
Sketch A Properly Labeled Titration Curve For The Che vrogue.co Equivalence Point Of The Titration Curve Sorting out some confusing terms. Nearer to the equivalence point,. equivalence point (v = 25 ml): a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. the equivalence point of a titration. As base is added to acid at the beginning of a titration, the ph rises very slowly. . Equivalence Point Of The Titration Curve.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Equivalence Point Of The Titration Curve the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to. Equivalence Point Of The Titration Curve.
From www.chegg.com
Solved What is the approximate pH at the equivalence point Equivalence Point Of The Titration Curve equivalence point (v = 25 ml): a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Nearer to the equivalence point,. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. As base is added to acid at the beginning. Equivalence Point Of The Titration Curve.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Equivalence Point Of The Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. Nearer to the equivalence point,. the equivalence point of a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. equivalence point (v = 25 ml): Sorting out. Equivalence Point Of The Titration Curve.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Equivalence Point Of The Titration Curve the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Sorting out some confusing terms. the equivalence point of a titration. equivalence point (v = 25 ml): a typical titration curve of a diprotic acid, oxalic acid, titrated with a. Equivalence Point Of The Titration Curve.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Equivalence Point Of The Titration Curve the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Nearer to the equivalence point,. As base is added to acid at the beginning of a titration, the ph rises very slowly. the equivalence point of a titration is the point at. Equivalence Point Of The Titration Curve.
From mungfali.com
Equivalence Points On Titration Graph Equivalence Point Of The Titration Curve the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. As base is added to acid at the beginning of a titration, the ph rises very slowly. the equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic. Equivalence Point Of The Titration Curve.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point Of The Titration Curve Nearer to the equivalence point,. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. equivalence point (v = 25 ml): the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or.. Equivalence Point Of The Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point Of The Titration Curve As base is added to acid at the beginning of a titration, the ph rises very slowly. equivalence point (v = 25 ml): Nearer to the equivalence point,. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. A drastic rise in. Equivalence Point Of The Titration Curve.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point Of The Titration Curve equivalence point (v = 25 ml): As base is added to acid at the beginning of a titration, the ph rises very slowly. the equivalence point of a titration. Sorting out some confusing terms. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. Equivalence Point Of The Titration Curve.
From mungfali.com
Endpoint Titration Curve Equivalence Point Of The Titration Curve the equivalence point of a titration. As base is added to acid at the beginning of a titration, the ph rises very slowly. equivalence point (v = 25 ml): the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. A drastic rise in ph is observed. Equivalence Point Of The Titration Curve.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Equivalence Point Of The Titration Curve As base is added to acid at the beginning of a titration, the ph rises very slowly. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. A drastic rise in ph is observed as the solution composition transitions from acidic to either. Equivalence Point Of The Titration Curve.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point Of The Titration Curve Sorting out some confusing terms. Nearer to the equivalence point,. equivalence point (v = 25 ml): the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. As base is added to acid at the beginning of a titration, the ph rises very. Equivalence Point Of The Titration Curve.
From gioilucnh.blob.core.windows.net
Titration Titrant Equivalence Point at Pamella blog Equivalence Point Of The Titration Curve the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Nearer to the equivalence point,. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. a typical titration curve of. Equivalence Point Of The Titration Curve.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Equivalence Point Of The Titration Curve the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. the equivalence point of a titration. As base is added. Equivalence Point Of The Titration Curve.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Equivalence Point Of The Titration Curve the equivalence point of a titration. As base is added to acid at the beginning of a titration, the ph rises very slowly. Nearer to the equivalence point,. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. the ph at the midpoint, the point halfway on the titration curve. Equivalence Point Of The Titration Curve.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Equivalence Point Of The Titration Curve the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Nearer to the equivalence point,. Sorting out some confusing terms. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. the equivalence point of. Equivalence Point Of The Titration Curve.
From fyoizufqj.blob.core.windows.net
Equivalence Point Amino Acid Titration at Christine Grosso blog Equivalence Point Of The Titration Curve As base is added to acid at the beginning of a titration, the ph rises very slowly. the equivalence point of a titration. Sorting out some confusing terms. Nearer to the equivalence point,. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. the ph at the midpoint, the point. Equivalence Point Of The Titration Curve.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Equivalence Point Of The Titration Curve As base is added to acid at the beginning of a titration, the ph rises very slowly. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. Nearer to the equivalence. Equivalence Point Of The Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Equivalence Point Of The Titration Curve the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Nearer to the equivalence point,. Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. a. Equivalence Point Of The Titration Curve.
From www.vrogue.co
How To Find Equivalence Point From Titration Data vrogue.co Equivalence Point Of The Titration Curve the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the ph at the midpoint, the point halfway on the titration. Equivalence Point Of The Titration Curve.
From hxeptiewa.blob.core.windows.net
Find Equivalence Point From Titration at Roger Cornelius blog Equivalence Point Of The Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. As base is added to acid at the beginning of a titration, the ph rises very slowly. Nearer to the equivalence point,. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid. Equivalence Point Of The Titration Curve.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Equivalence Point Of The Titration Curve the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. the equivalence point of a titration. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. equivalence point (v. Equivalence Point Of The Titration Curve.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Equivalence Point Of The Titration Curve Sorting out some confusing terms. Nearer to the equivalence point,. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. . Equivalence Point Of The Titration Curve.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Equivalence Point Of The Titration Curve the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Nearer to the equivalence point,. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the ph at the midpoint, the point halfway on the titration. Equivalence Point Of The Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point Of The Titration Curve the equivalence point of a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. Nearer to the equivalence point,. equivalence point (v = 25 ml): the. Equivalence Point Of The Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Equivalence Point Of The Titration Curve the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. As base is added to acid at the beginning of a titration, the ph rises very slowly. Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition. Equivalence Point Of The Titration Curve.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Equivalence Point Of The Titration Curve the equivalence point of a titration. As base is added to acid at the beginning of a titration, the ph rises very slowly. Sorting out some confusing terms. Nearer to the equivalence point,. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. A drastic rise in. Equivalence Point Of The Titration Curve.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Equivalence Point Of The Titration Curve Nearer to the equivalence point,. Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. As base is added to acid at the beginning of a titration, the ph rises very slowly. the equivalence point of a titration is the point at which. Equivalence Point Of The Titration Curve.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Equivalence Point Of The Titration Curve Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. equivalence point (v = 25 ml): As base is added to. Equivalence Point Of The Titration Curve.
From mungfali.com
Titration Curve Labeled Equivalence Point Of The Titration Curve As base is added to acid at the beginning of a titration, the ph rises very slowly. Nearer to the equivalence point,. the equivalence point of a titration. Sorting out some confusing terms. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A drastic rise in ph is observed as. Equivalence Point Of The Titration Curve.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Equivalence Point Of The Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or. Nearer to the equivalence point,. the equivalence point of a titration. Equivalence Point Of The Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Equivalence Point Of The Titration Curve the equivalence point of a titration. Nearer to the equivalence point,. the equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A drastic rise in ph is observed as. Equivalence Point Of The Titration Curve.