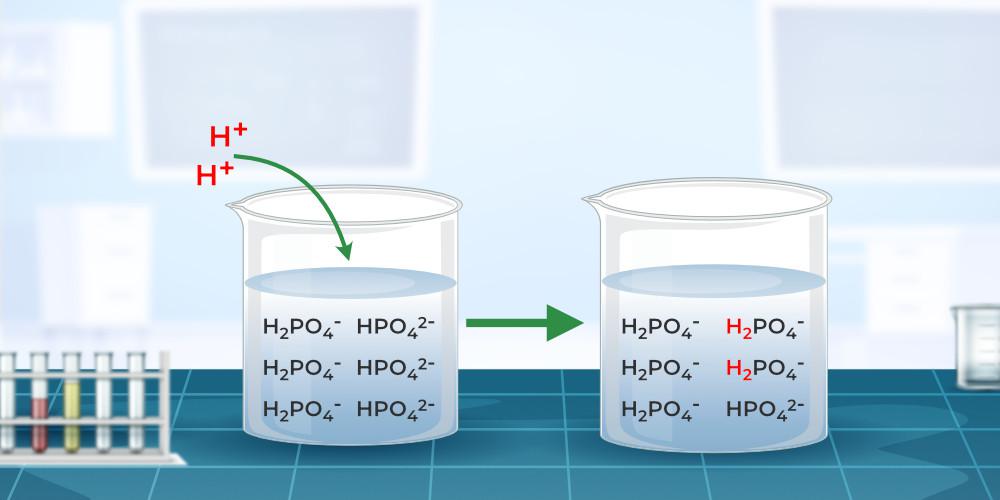
How To Make Ph 2 Buffer Solution . buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. There are two ways of calculating the ph of a buffer solution; a buffer is a solution that maintains a constant ph when an external acid or base is added to it. suppose we needed to make a buffer solution with a ph of 2.11. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. the ph of a buffer solution. In the previous post, we talked about the ph of buffer solutions and the two approaches to. The equilibrium approach and the one using the. This is done by having an internal acid and base within the. In the first case, we would try and find a weak acid with a. preparing a buffer with a specific ph.
from www.geeksforgeeks.org
This is done by having an internal acid and base within the. In the first case, we would try and find a weak acid with a. In the previous post, we talked about the ph of buffer solutions and the two approaches to. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. the ph of a buffer solution. suppose we needed to make a buffer solution with a ph of 2.11. There are two ways of calculating the ph of a buffer solution; standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. preparing a buffer with a specific ph.
Buffer Solution Definition, Types, Formula, Examples, and FAQs
How To Make Ph 2 Buffer Solution buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. preparing a buffer with a specific ph. This is done by having an internal acid and base within the. In the first case, we would try and find a weak acid with a. The equilibrium approach and the one using the. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. There are two ways of calculating the ph of a buffer solution; suppose we needed to make a buffer solution with a ph of 2.11. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. In the previous post, we talked about the ph of buffer solutions and the two approaches to. the ph of a buffer solution.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. suppose we needed to make a buffer solution with a ph of 2.11. This is done by having an internal acid and base within the. the ph of a buffer solution. a buffer is a solution that maintains a constant ph when an external acid or base is added. How To Make Ph 2 Buffer Solution.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 How To Make Ph 2 Buffer Solution This is done by having an internal acid and base within the. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. In the first case, we would try and find a weak acid with a. a buffer is a solution that maintains a constant ph when an external acid. How To Make Ph 2 Buffer Solution.
From www.youtube.com
How to Calculate PH of Buffer Solution YouTube How To Make Ph 2 Buffer Solution The equilibrium approach and the one using the. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. There are two ways of calculating the ph of a buffer solution; preparing a buffer with a specific ph. buffers can be made by combining h 3 po 4. How To Make Ph 2 Buffer Solution.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps How To Make Ph 2 Buffer Solution In the first case, we would try and find a weak acid with a. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. The equilibrium approach and the one using the. In the previous post, we talked about the ph of buffer solutions and the two approaches to. buffers. How To Make Ph 2 Buffer Solution.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. There are two ways of calculating the ph of a buffer solution; standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. a buffer is a solution that maintains a constant ph when an external acid or base is added to it.. How To Make Ph 2 Buffer Solution.
From onlinereagents.co.uk
Buffer solution pH 2.0 Online Reagents How To Make Ph 2 Buffer Solution There are two ways of calculating the ph of a buffer solution; standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. This is done by having an internal acid and base within the. The equilibrium approach and the one using the. In the first case, we would try and find. How To Make Ph 2 Buffer Solution.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. the ph of a buffer solution. In the previous post, we talked about the ph of buffer solutions and the two approaches to. buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo. How To Make Ph 2 Buffer Solution.
From www.youtube.com
How to calculate the pH of a buffer solution YouTube How To Make Ph 2 Buffer Solution This is done by having an internal acid and base within the. There are two ways of calculating the ph of a buffer solution; preparing a buffer with a specific ph. the ph of a buffer solution. a buffer is a solution that maintains a constant ph when an external acid or base is added to it.. How To Make Ph 2 Buffer Solution.
From www.fishersci.fi
Technical Buffer Solution pH 2, WTW Fisher Scientific How To Make Ph 2 Buffer Solution buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. suppose we needed to. How To Make Ph 2 Buffer Solution.
From exydsrovs.blob.core.windows.net
How To Use Buffer Solution Ph at Clint Stacey blog How To Make Ph 2 Buffer Solution There are two ways of calculating the ph of a buffer solution; standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. This is done by having an internal acid and base within the. a buffer is a solution that maintains a constant ph when an external acid or base. How To Make Ph 2 Buffer Solution.
From www.youtube.com
How to Make and pH Buffers YouTube How To Make Ph 2 Buffer Solution buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. In the first case, we would try and find a weak acid with a. The equilibrium approach and the one using the. This is done by. How To Make Ph 2 Buffer Solution.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 How To Make Ph 2 Buffer Solution buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. standard buffer solutions for. How To Make Ph 2 Buffer Solution.
From exyfwexcg.blob.core.windows.net
What Is A Buffer In Ph at Katie Smith blog How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. There are two ways of calculating the ph of a buffer solution; The equilibrium approach and the one using the. This is done by having an internal acid and base within the. In the previous post, we talked about the ph of buffer solutions and the two approaches to. the ph. How To Make Ph 2 Buffer Solution.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs How To Make Ph 2 Buffer Solution This is done by having an internal acid and base within the. suppose we needed to make a buffer solution with a ph of 2.11. In the first case, we would try and find a weak acid with a. In the previous post, we talked about the ph of buffer solutions and the two approaches to. the ph. How To Make Ph 2 Buffer Solution.
From www.youtube.com
pH of Buffer Solution (Example) YouTube How To Make Ph 2 Buffer Solution The equilibrium approach and the one using the. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. In the first case, we would try and find a weak acid with a. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by. How To Make Ph 2 Buffer Solution.
From design.udlvirtual.edu.pe
Designing A Buffer Solution With A Specific Ph Design Talk How To Make Ph 2 Buffer Solution a buffer is a solution that maintains a constant ph when an external acid or base is added to it. suppose we needed to make a buffer solution with a ph of 2.11. The equilibrium approach and the one using the. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by. How To Make Ph 2 Buffer Solution.
From www.chegg.com
Solved Calculating the pH of a Buffer Solution To prepare a How To Make Ph 2 Buffer Solution the ph of a buffer solution. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. In the previous post, we talked about the ph of buffer solutions and the two approaches to. buffers can be made by combining h 3 po 4 and h 2 po 4 −,. How To Make Ph 2 Buffer Solution.
From www.youtube.com
pH of buffer solutions the HendersonHasselbalch equation. YouTube How To Make Ph 2 Buffer Solution In the previous post, we talked about the ph of buffer solutions and the two approaches to. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. There are two. How To Make Ph 2 Buffer Solution.
From www.youtube.com
Buffer Solutions PH Calculations YouTube How To Make Ph 2 Buffer Solution standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. This is done by having an internal acid and base within the. buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4. How To Make Ph 2 Buffer Solution.
From scienceinhydroponics.com
Preparing your own buffer solutions for pH calibration Science in How To Make Ph 2 Buffer Solution a buffer is a solution that maintains a constant ph when an external acid or base is added to it. There are two ways of calculating the ph of a buffer solution; preparing a buffer with a specific ph. In the previous post, we talked about the ph of buffer solutions and the two approaches to. In the. How To Make Ph 2 Buffer Solution.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions How To Make Ph 2 Buffer Solution In the first case, we would try and find a weak acid with a. suppose we needed to make a buffer solution with a ph of 2.11. the ph of a buffer solution. This is done by having an internal acid and base within the. preparing a buffer with a specific ph. buffers can be made. How To Make Ph 2 Buffer Solution.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. There are two ways of calculating the ph of a buffer solution; buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. In the previous post, we talked. How To Make Ph 2 Buffer Solution.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How To Make Ph 2 Buffer Solution buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. In the first case, we would try and find a weak acid with a. preparing a buffer with a specific ph. In the previous post,. How To Make Ph 2 Buffer Solution.
From www.youtube.com
how to prepare a buffer with a particular pH YouTube How To Make Ph 2 Buffer Solution buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. The equilibrium approach and the one using the. suppose we needed to make a buffer solution with a ph of 2.11. This is done by. How To Make Ph 2 Buffer Solution.
From www.youtube.com
Find the pH of a Buffer after Adding HCl YouTube How To Make Ph 2 Buffer Solution There are two ways of calculating the ph of a buffer solution; buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. the ph of a buffer solution. standard buffer solutions for various ranges. How To Make Ph 2 Buffer Solution.
From www.scribd.com
How Do I Prepare a Phosphate Buffer Solution With a Specific PH Ph How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. In the previous post, we talked about the ph of buffer solutions and the two approaches to. This is done by having an internal acid and base within the. The equilibrium approach and the one using the. There are two ways of calculating the ph of a buffer solution; a buffer. How To Make Ph 2 Buffer Solution.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 How To Make Ph 2 Buffer Solution This is done by having an internal acid and base within the. the ph of a buffer solution. There are two ways of calculating the ph of a buffer solution; preparing a buffer with a specific ph. In the previous post, we talked about the ph of buffer solutions and the two approaches to. standard buffer solutions. How To Make Ph 2 Buffer Solution.
From giokrstgg.blob.core.windows.net
Buffer Solution Equilibrium at Jacqueline Scott blog How To Make Ph 2 Buffer Solution suppose we needed to make a buffer solution with a ph of 2.11. In the previous post, we talked about the ph of buffer solutions and the two approaches to. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. preparing a buffer with a specific ph. This is. How To Make Ph 2 Buffer Solution.
From exydsrovs.blob.core.windows.net
How To Use Buffer Solution Ph at Clint Stacey blog How To Make Ph 2 Buffer Solution There are two ways of calculating the ph of a buffer solution; the ph of a buffer solution. preparing a buffer with a specific ph. In the first case, we would try and find a weak acid with a. buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2. How To Make Ph 2 Buffer Solution.
From chemguru.sg
Calculate pH of Buffer Solution How To Make Ph 2 Buffer Solution In the previous post, we talked about the ph of buffer solutions and the two approaches to. buffers can be made by combining h 3 po 4 and h 2 po 4 −, h 2 po 4 − and hpo 4 2−, and hpo 4 2− and po 4 3−. This is done by having an internal acid and. How To Make Ph 2 Buffer Solution.
From www.chegg.com
Solved Calculating the pH of a Buffer Solution To prepare a How To Make Ph 2 Buffer Solution The equilibrium approach and the one using the. preparing a buffer with a specific ph. suppose we needed to make a buffer solution with a ph of 2.11. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. There are two ways of calculating the ph of a buffer. How To Make Ph 2 Buffer Solution.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy How To Make Ph 2 Buffer Solution There are two ways of calculating the ph of a buffer solution; preparing a buffer with a specific ph. suppose we needed to make a buffer solution with a ph of 2.11. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. This is done by having. How To Make Ph 2 Buffer Solution.
From labmonk.com
Preparation of Buffer Solutions and Measurement of pH Labmonk How To Make Ph 2 Buffer Solution In the first case, we would try and find a weak acid with a. In the previous post, we talked about the ph of buffer solutions and the two approaches to. standard buffer solutions for various ranges of ph values 1.2 to 10.0 may be prepared by appropriate combinations. This is done by having an internal acid and base. How To Make Ph 2 Buffer Solution.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide How To Make Ph 2 Buffer Solution In the previous post, we talked about the ph of buffer solutions and the two approaches to. the ph of a buffer solution. a buffer is a solution that maintains a constant ph when an external acid or base is added to it. The equilibrium approach and the one using the. preparing a buffer with a specific. How To Make Ph 2 Buffer Solution.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube How To Make Ph 2 Buffer Solution preparing a buffer with a specific ph. This is done by having an internal acid and base within the. suppose we needed to make a buffer solution with a ph of 2.11. the ph of a buffer solution. The equilibrium approach and the one using the. In the previous post, we talked about the ph of buffer. How To Make Ph 2 Buffer Solution.