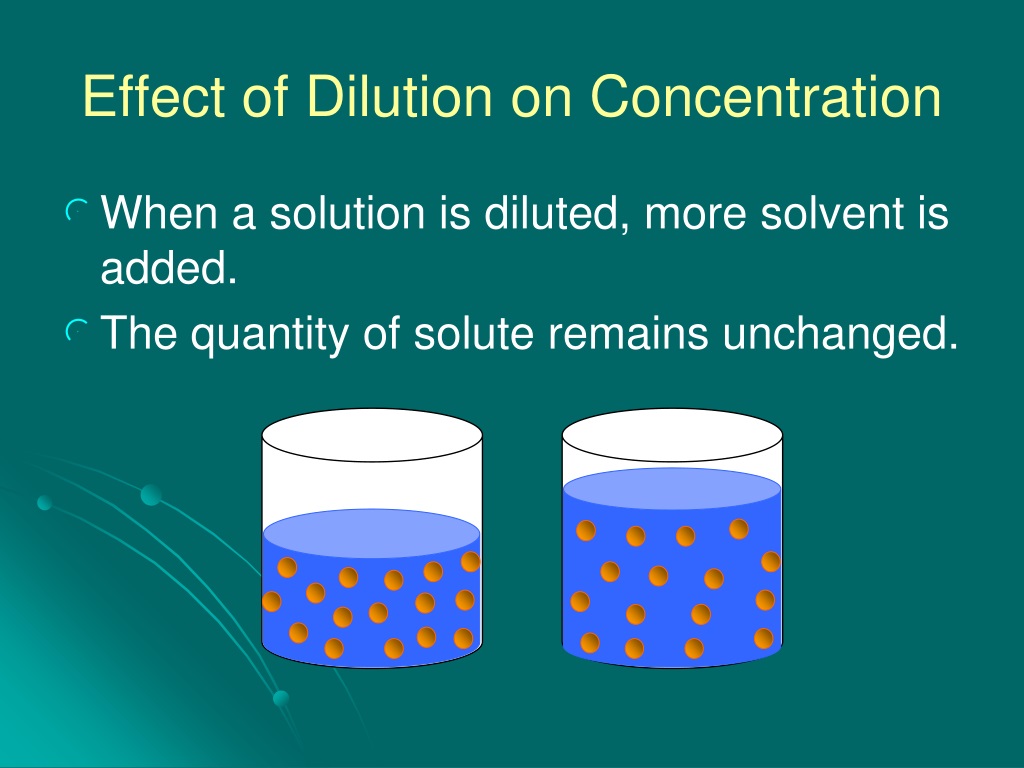
Dilution And Concentration . Dilution is a process used to lower the concentration of the original solution by adding more solvent. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Often, a worker will need to change the concentration of a solution by changing the. See examples, exercises and applications in medicine and chemistry. Learn how to dilute and concentrate solutions. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume.
from www.slideserve.com
Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Often, a worker will need to change the concentration of a solution by changing the. See examples, exercises and applications in medicine and chemistry. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions:
PPT Volumetric Analysis PowerPoint Presentation, free download ID
Dilution And Concentration State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. See examples, exercises and applications in medicine and chemistry. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a solution by changing the. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions.
From studylib.net
Concentration and Dilution Dilution And Concentration Learn how to dilute and concentrate solutions. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilution And Concentration.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution And Concentration Learn how to calculate the new concentration or volume of a solution after dilution or concentration. See examples, exercises and applications in medicine and chemistry. Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process. Dilution And Concentration.
From www.youtube.com
Dilution and Concentration Calculations in Pharmacy 5 Key Examples Dilution And Concentration Learn how to calculate the new concentration or volume of a solution after dilution or concentration. State whether the concentration of a solution is directly or indirectly proportional to its volume. See examples, exercises and applications in medicine and chemistry. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process. Dilution And Concentration.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. State whether the concentration of a solution is directly or indirectly proportional to its volume. See examples, exercises and applications in medicine and chemistry. In. Dilution And Concentration.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution And Concentration Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Learn how to dilute and. Dilution And Concentration.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution And Concentration In both dilution and concentration,. Often, a worker will need to change the concentration of a solution by changing the. See examples, exercises and applications in medicine and chemistry. Learn how to dilute and concentrate solutions. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is the addition of. Dilution And Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: See examples, exercises and applications in medicine and chemistry. State whether the concentration of a solution is directly or. Dilution And Concentration.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution And Concentration Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Diluting solutions means increasing the volume of a solution and decreasing the concentration. Dilution And Concentration.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution And Concentration Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. See examples, exercises and applications in medicine and chemistry. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering. Dilution And Concentration.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Dilution And Concentration Learn how to dilute and concentrate solutions. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilution And Concentration.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution And Concentration Learn how to calculate the new concentration or volume of a solution after dilution or concentration. In both dilution and concentration,. Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which. Dilution And Concentration.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution And Concentration Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how. Dilution And Concentration.
From www.rxcalculations.com
Dilution and Concentration Dilution And Concentration Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to. Dilution And Concentration.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution And Concentration Learn how to dilute and concentrate solutions. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to. Dilution And Concentration.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. See examples, exercises and applications in medicine and chemistry. In both dilution and concentration,.. Dilution And Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution And Concentration Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the. Dilution And Concentration.
From www.differencebetween.com
Difference Between Dilution and Concentration Compare the Difference Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. In. Dilution And Concentration.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID3874312 Dilution And Concentration Dilution is a process used to lower the concentration of the original solution by adding more solvent. See examples, exercises and applications in medicine and chemistry. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. In both dilution and concentration,. Diluting solutions means increasing the volume of a solution and decreasing the concentration. Dilution And Concentration.
From differencebtw.com
Dilution vs. Concentration Know the Difference Dilution And Concentration Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In. Dilution And Concentration.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution And Concentration Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of. Dilution And Concentration.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution And Concentration State whether the concentration of a solution is directly or indirectly proportional to its volume. See examples, exercises and applications in medicine and chemistry. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. Diluting solutions means increasing the volume of a solution and decreasing the concentration without. Dilution And Concentration.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution And Concentration State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is a process used to lower the concentration of. Dilution And Concentration.
From sapiascience.wordpress.com
Dilution and Concentration The Sapia Science Blog Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. See examples, exercises and applications in medicine and chemistry. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Since the amount of moles stays the same we can use the concentration equation to create. Dilution And Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution And Concentration In both dilution and concentration,. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. State whether the. Dilution And Concentration.
From www.slideserve.com
PPT Dilutions and concentrations Lab 7 PowerPoint Presentation, free Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a solution by changing the. Diluting solutions means increasing the volume of a solution and decreasing the. Dilution And Concentration.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution And Concentration Learn how to calculate the new concentration or volume of a solution after dilution or concentration. Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions:. Dilution And Concentration.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution And Concentration See examples, exercises and applications in medicine and chemistry. Often, a worker will need to change the concentration of a solution by changing the. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to. Dilution And Concentration.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution And Concentration Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. See examples, exercises and applications. Dilution And Concentration.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilution And Concentration Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Often, a worker will need to change the concentration of a solution by changing the. Learn how to dilute and concentrate solutions. See examples, exercises. Dilution And Concentration.
From www.coursehero.com
[Solved] Exercise 3 Concentration, Solution, and Dilution Data Table 8 Dilution And Concentration Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a solution by changing the. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. See examples, exercises and applications in medicine and chemistry. Diluting solutions means. Dilution And Concentration.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilution And Concentration Learn how to dilute and concentrate solutions. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilution And Concentration.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution And Concentration Learn how to dilute and concentrate solutions. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to. Dilution And Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution And Concentration Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume. See examples, exercises and applications in medicine and chemistry. Learn how to calculate the new concentration. Dilution And Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution And Concentration Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to calculate the new concentration or volume of a solution after dilution or concentration. State whether the concentration of a solution is directly or indirectly proportional to its volume. In both dilution and concentration,. See examples, exercises and applications in medicine. Dilution And Concentration.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution And Concentration Learn how to dilute and concentrate solutions. Since the amount of moles stays the same we can use the concentration equation to create an equation for dilutions: See examples, exercises and applications in medicine and chemistry. Diluting solutions means increasing the volume of a solution and decreasing the concentration without altering the amount of moles. Often, a worker will need. Dilution And Concentration.