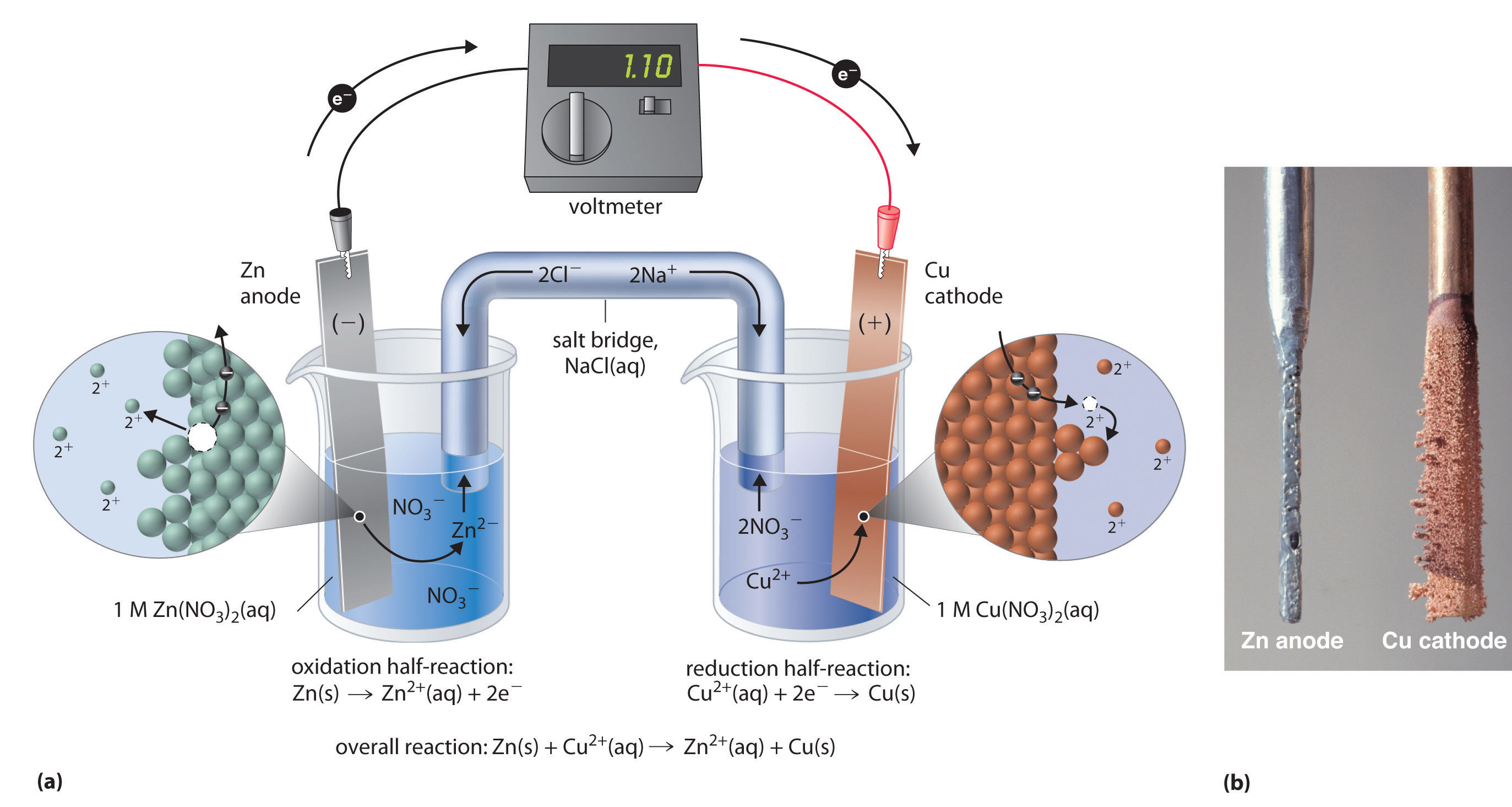
Fuel Cell Electrochemical Reaction . A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Fuel cells are efficient energy converters, based on electrochemical principles. Oxygen is supplied to a similar electrode except that the catalyst is. It offers high efficiency and zero emissions. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. They convert the chemical energy (heating value) of a fuel. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst.
from saylordotorg.github.io
A fuel cell resembles a battery in. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. They convert the chemical energy (heating value) of a fuel. It offers high efficiency and zero emissions. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are efficient energy converters, based on electrochemical principles.
Electrochemistry
Fuel Cell Electrochemical Reaction Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. It offers high efficiency and zero emissions. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. They convert the chemical energy (heating value) of a fuel. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Fuel cells are efficient energy converters, based on electrochemical principles.
From www.betase.nl
Electrochemistry of the fuel cell Betase BV Fuel Cell Electrochemical Reaction A fuel cell resembles a battery in. It offers high efficiency and zero emissions. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. They convert the chemical energy (heating value) of a fuel. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Schematic of a microbial fuel cell (a), microbial electrolysis cell Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Hydrogen enters the cell through a porous. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
1 Fuel Cell Reaction through and external circuit, thus creating Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Oxygen is supplied to a similar electrode except that the catalyst is. It offers high. Fuel Cell Electrochemical Reaction.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell Electrochemical Reaction A fuel cell resembles a battery in. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. It offers high efficiency and zero emissions. Fuel cells are efficient energy converters, based on electrochemical. Fuel Cell Electrochemical Reaction.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to a similar electrode except that the catalyst is. Fuel cells are efficient energy converters, based on electrochemical principles. They convert the chemical energy (heating value) of a fuel. Fuel cell, any of. Fuel Cell Electrochemical Reaction.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Fuel Cell Electrochemical Reaction Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Oxygen is. Fuel Cell Electrochemical Reaction.
From opentextbc.ca
Applications of Redox Reactions Voltaic Cells Introductory Chemistry Fuel Cell Electrochemical Reaction Fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Oxygen is supplied to. Fuel Cell Electrochemical Reaction.
From www.youtube.com
Fuel Cell (0601) Thermodynamics Electrochem Reaction YouTube Fuel Cell Electrochemical Reaction Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell resembles a battery in. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. Fuel Cell Electrochemical Reaction.
From www.slideserve.com
PPT How Fuel Cells Work PowerPoint Presentation, free download ID Fuel Cell Electrochemical Reaction It offers high efficiency and zero emissions. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell is an electrochemical cell in which a fuel donates electrons at. Fuel Cell Electrochemical Reaction.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel Cell Electrochemical Reaction A fuel cell resembles a battery in. It offers high efficiency and zero emissions. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions.. Fuel Cell Electrochemical Reaction.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Fuel Cell Electrochemical Reaction It offers high efficiency and zero emissions. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells are efficient energy converters, based on electrochemical principles. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Fuel cell, any of. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Layer structure and electrochemical reactions of PEM fuel cell Fuel Cell Electrochemical Reaction It offers high efficiency and zero emissions. Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. They convert the chemical energy (heating value) of a fuel. Fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell is an electrochemical. Fuel Cell Electrochemical Reaction.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Fuel Cell Electrochemical Reaction A fuel cell resembles a battery in. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Hydrogen enters the cell through a porous carbon. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Electrochemical reaction of PEM fuel cell electrode (cathode side) to Fuel Cell Electrochemical Reaction It offers high efficiency and zero emissions. They convert the chemical energy (heating value) of a fuel. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to a similar electrode except that the catalyst is. Fuel cells are efficient energy converters, based. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Schematics of a) Alkaline Fuel Cell (AFC), b) Phosphoric Acid Fuel Cell Fuel Cell Electrochemical Reaction Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to. Fuel Cell Electrochemical Reaction.
From www.preprints.org
Educational Model of Electric Potential, Electrochemical Reactions, and Fuel Cell Electrochemical Reaction Fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cell, any of a class of devices that. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
15 Fuel cell types and reactions. Download Scientific Diagram Fuel Cell Electrochemical Reaction Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. They convert the chemical energy (heating value) of a fuel. It offers high efficiency and zero emissions. A fuel cell resembles a battery in. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Electrochemically, rather than being. Fuel Cell Electrochemical Reaction.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Electrochemically, rather than being burned, so. Fuel Cell Electrochemical Reaction.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. It offers high efficiency and zero emissions. They convert the chemical energy (heating value) of a fuel. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity. Fuel Cell Electrochemical Reaction.
From mmerevise.co.uk
Commercial Applications of Electrochemical Cells MME Fuel Cell Electrochemical Reaction Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. It offers high efficiency and zero emissions. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Fuel cell, any of a class of devices that convert the chemical energy of a. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Electrochemical reactions in a fuel cell with (A) H 2 O 2 and (B) CH 4 Fuel Cell Electrochemical Reaction Fuel cells are efficient energy converters, based on electrochemical principles. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell resembles a battery in. A fuel cell is an electrochemical cell in which a fuel. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Schematic diagram of the electrochemical CO2 reduction reaction system Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell resembles a battery in. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are efficient energy converters, based. Fuel Cell Electrochemical Reaction.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. They convert the chemical energy (heating value) of a fuel. A fuel cell resembles. Fuel Cell Electrochemical Reaction.
From www.chegg.com
Solved Hydrogen Fuel Cell Fuel cells are electrochemical Fuel Cell Electrochemical Reaction Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are efficient energy converters, based on electrochemical principles. Oxygen is supplied to a similar electrode except that the catalyst is. It offers high efficiency and zero emissions. A fuel cell is an electrochemical cell in which. Fuel Cell Electrochemical Reaction.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cell Electrochemical Reaction They convert the chemical energy (heating value) of a fuel. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is an electrochemical cell that generates electrical energy from fuel. Fuel Cell Electrochemical Reaction.
From www.mdpi.com
Energies Free FullText Principles and Materials Aspects of Direct Fuel Cell Electrochemical Reaction Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. They convert the chemical energy (heating value) of a fuel. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains. Fuel Cell Electrochemical Reaction.
From saylordotorg.github.io
Electrochemistry Fuel Cell Electrochemical Reaction Fuel cells are efficient energy converters, based on electrochemical principles. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. Fuel Cell Electrochemical Reaction.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Fuel Cell Electrochemical Reaction Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. They convert the chemical. Fuel Cell Electrochemical Reaction.
From www.youtube.com
EC24/TN12th STD/Fuel Cells H2O2 Functioning of Fuel cells/Secondary Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. It offers high efficiency and zero emissions. Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Electrochemically, rather than being burned, so the reaction takes. Fuel Cell Electrochemical Reaction.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell resembles a battery in. It offers high efficiency and zero emissions. They convert the chemical energy (heating value) of a fuel. Oxygen is supplied to a similar electrode except that the catalyst is. Fuel cells are efficient energy converters, based. Fuel Cell Electrochemical Reaction.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell Electrochemical Reaction A fuel cell resembles a battery in. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. It offers high efficiency and zero emissions. Oxygen is supplied to a similar electrode except. Fuel Cell Electrochemical Reaction.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Cell Electrochemical Reaction Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the. Fuel Cell Electrochemical Reaction.
From dokumen.tips
(PPT) Fuel cells An electrochemical conversion device Chemical Fuel Cell Electrochemical Reaction Fuel cells are efficient energy converters, based on electrochemical principles. Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel. Fuel Cell Electrochemical Reaction.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Fuel Cell Electrochemical Reaction A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells are efficient energy converters, based on electrochemical principles. Oxygen is supplied to a similar electrode except that the catalyst is. They convert the chemical energy (heating value) of a fuel. Fuel cell, any of. Fuel Cell Electrochemical Reaction.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Fuel Cell Electrochemical Reaction Electrochemically, rather than being burned, so the reaction takes place at a lower temperature than if it was to be burned. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. It offers high efficiency and zero emissions. Fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell is an electrochemical cell. Fuel Cell Electrochemical Reaction.