What Bonds With Chlorine . The reason that the two chlorine atoms stick together is that the. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. Unlike fluorine, chlorine can form. The two chlorine atoms are said to be joined by a covalent bond. A chlorine atom has 7 electrons in its outer shell. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Chlorine is in group 7 of the periodic table. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. The two chlorine atoms are said to be joined by a covalent bond.
from chem.libretexts.org
However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Chlorine is in group 7 of the periodic table. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. The two chlorine atoms are said to be joined by a covalent bond. The two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. Unlike fluorine, chlorine can form. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2.
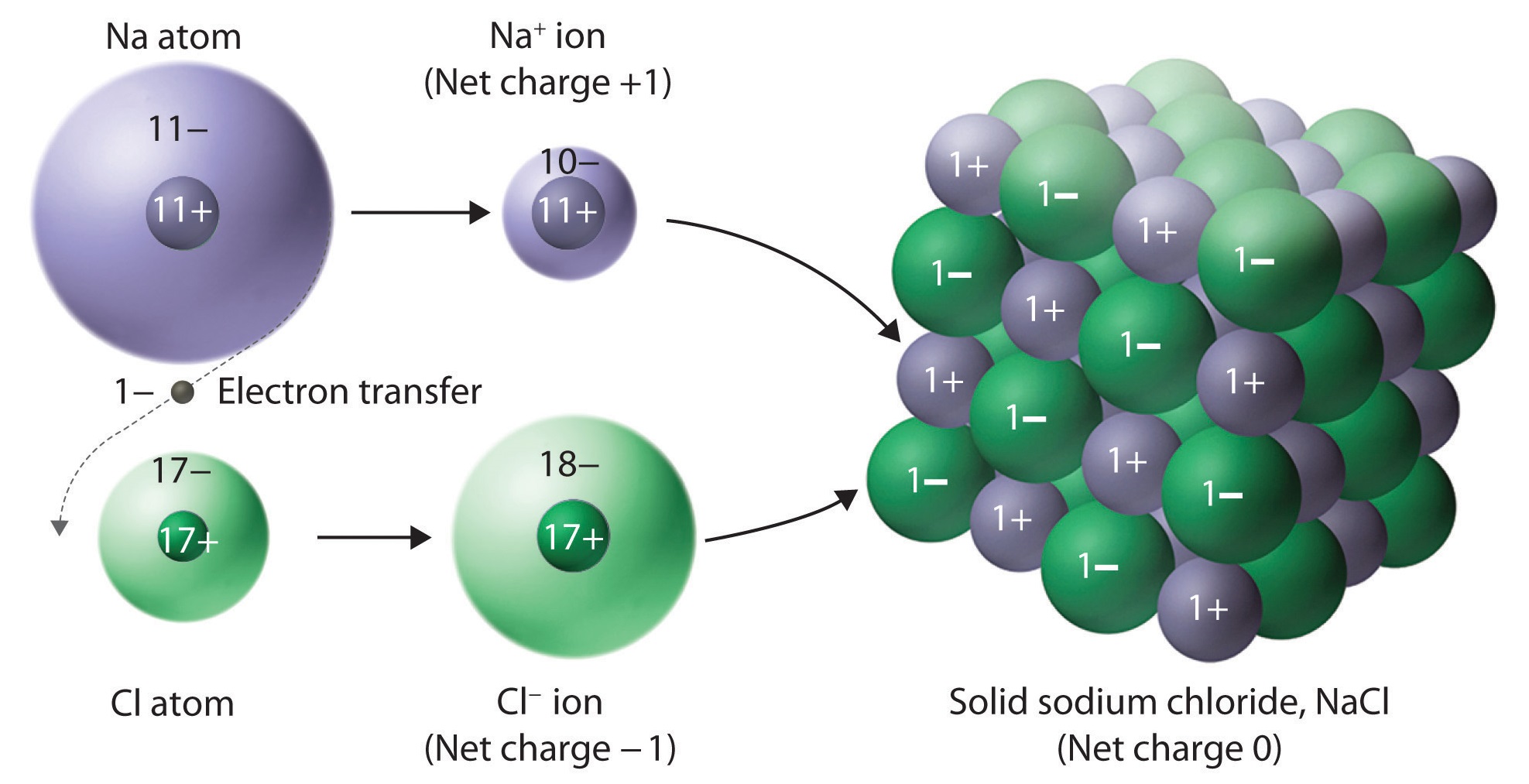
Ionic Solids Chemistry LibreTexts
What Bonds With Chlorine Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. A chlorine atom has 7 electrons in its outer shell. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Chlorine is in group 7 of the periodic table. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. Unlike fluorine, chlorine can form. The two chlorine atoms are said to be joined by a covalent bond. The two chlorine atoms are said to be joined by a covalent bond. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the.
From www.youtube.com
Covalent Bond of Hydrogen , Chlorine and Hydro Chloride Molecule What Bonds With Chlorine The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. Chlorine is in group 7 of the periodic table. The two chlorine atoms are said to be joined by a covalent bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an. What Bonds With Chlorine.
From www.thesciencehive.co.uk
Covalent bonding (GCSE) — the science sauce What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to. What Bonds With Chlorine.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The reason that the two chlorine atoms stick together is that the. However, there are two key features with regard to chlorine’s bonding that differentiates it from. What Bonds With Chlorine.
From www.youtube.com
Covalent Bonding Chlorine Gas YouTube What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the. A chlorine atom has 7 electrons in its outer shell. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. Chlorine is in group 7 of the periodic table. The two chlorine atoms are said. What Bonds With Chlorine.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the. The two chlorine atoms are said to be joined by a covalent bond. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The two chlorine atoms are said to be joined by a covalent bond. Unlike fluorine, chlorine can form. The. What Bonds With Chlorine.
From www.shutterstock.com
Single Covalent Bond Chlorine Molecule Stock Vector (Royalty Free What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. Unlike fluorine, chlorine can form. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The two chlorine atoms are said to be joined by a covalent. What Bonds With Chlorine.
From www.chemistrylearner.com
Beryllium Chloride Facts, Formula, Properties, Uses, Safety Data What Bonds With Chlorine The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. Chlorine is in group 7 of the periodic table. Two chlorine atoms will each share one electron. What Bonds With Chlorine.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells What Bonds With Chlorine However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. Chlorine is in group 7 of the periodic table. The slideshow shows. What Bonds With Chlorine.
From thesciencecore.blogspot.com
covalent bond definition, properties and examples The Science Core What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the. Chlorine has a far larger electronegativity. What Bonds With Chlorine.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Sodium Chloride Stock Illustration What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Chlorine is in group 7 of the periodic table. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form. What Bonds With Chlorine.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross What Bonds With Chlorine Chlorine is in group 7 of the periodic table. Unlike fluorine, chlorine can form. The two chlorine atoms are said to be joined by a covalent bond. A chlorine atom has 7 electrons in its outer shell. The two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is. What Bonds With Chlorine.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image What Bonds With Chlorine However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The two chlorine atoms are said to be joined by a covalent bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Unlike fluorine, chlorine can form. Chlorine is in group 7 of. What Bonds With Chlorine.
From slideplayer.com
Chemical Bonds Section ppt download What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The two chlorine atoms are said to be joined by a covalent bond. The two chlorine atoms are said to be joined by a covalent bond. The reason. What Bonds With Chlorine.
From www.slideserve.com
PPT Molecules, Compounds & Chemical Equations PowerPoint Presentation What Bonds With Chlorine A chlorine atom has 7 electrons in its outer shell. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. The reason that the two chlorine atoms. What Bonds With Chlorine.
From slideplayer.com
How do elements bond?. ppt download What Bonds With Chlorine Chlorine is in group 7 of the periodic table. The two chlorine atoms are said to be joined by a covalent bond. A chlorine atom has 7 electrons in its outer shell. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. Two chlorine atoms will each share one electron. What Bonds With Chlorine.
From slideplayer.com
Chapter 22 Chemical Bonds Types of Bonds ppt download What Bonds With Chlorine Unlike fluorine, chlorine can form. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. The reason that the two chlorine atoms stick together is that the. Two chlorine atoms will. What Bonds With Chlorine.
From animalia-life.club
Lewis Dot Structure For Chlorine What Bonds With Chlorine However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the. A chlorine atom has 7 electrons in its outer shell. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. Chlorine is. What Bonds With Chlorine.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Chlorine has a far larger electronegativity and so pulls the bonding electrons. What Bonds With Chlorine.
From techiescientist.com
ClO2 Lewis Structure, Geometry, Hybridization, and Polarity What Bonds With Chlorine Chlorine is in group 7 of the periodic table. A chlorine atom has 7 electrons in its outer shell. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus. What Bonds With Chlorine.
From pixels.com
Polar Bond In Hydrogen Chloride Molecule Photograph by What Bonds With Chlorine A chlorine atom has 7 electrons in its outer shell. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The two chlorine atoms are said to be joined by a covalent bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining. What Bonds With Chlorine.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning What Bonds With Chlorine However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Unlike fluorine, chlorine can form. The two chlorine atoms are said to. What Bonds With Chlorine.
From guidelistpstrapanning.z14.web.core.windows.net
Diagram Ionic Bond What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. Two. What Bonds With Chlorine.
From gerahellme.weebly.com
__TOP__ How Many Covalent Bonds Can Chlorine Form What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. The two chlorine atoms are said to be joined by a. What Bonds With Chlorine.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica What Bonds With Chlorine Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The two chlorine atoms are said to be joined by a covalent bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. The reason that the two chlorine atoms. What Bonds With Chlorine.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. The slideshow shows a. What Bonds With Chlorine.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts What Bonds With Chlorine Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Unlike fluorine, chlorine can. What Bonds With Chlorine.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. The two chlorine atoms are said to be joined by a covalent bond. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. A chlorine atom has. What Bonds With Chlorine.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare What Bonds With Chlorine The reason that the two chlorine atoms stick together is that the. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The two chlorine atoms are said to be joined by a covalent bond. Chlorine is in group 7 of the periodic table. A chlorine atom has 7 electrons in its outer shell.. What Bonds With Chlorine.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The reason that the two chlorine atoms stick together is that the. A chlorine atom has 7 electrons in its outer shell. Unlike fluorine, chlorine can form.. What Bonds With Chlorine.
From fineartamerica.com
Bond Formation In Chlorine Molecule Photograph by What Bonds With Chlorine Unlike fluorine, chlorine can form. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. A chlorine atom has 7 electrons in its outer shell. The two chlorine atoms are. What Bonds With Chlorine.
From animalia-life.club
Lewis Dot Structure For Chlorine What Bonds With Chlorine The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The two chlorine atoms are said to be joined by a covalent bond. A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the periodic table. Chlorine has a far larger electronegativity and so. What Bonds With Chlorine.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce What Bonds With Chlorine Unlike fluorine, chlorine can form. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to form hydrogen chloride. The two chlorine atoms are said to be joined by a covalent bond. The reason. What Bonds With Chlorine.
From slideplayer.com
Chapter 21 The Nature of Matter ppt download What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the. Chlorine is in group 7 of the periodic table. The two chlorine atoms are said to be joined by a covalent bond. A chlorine atom has 7 electrons in its outer shell. Unlike fluorine, chlorine. What Bonds With Chlorine.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's What Bonds With Chlorine The two chlorine atoms are said to be joined by a covalent bond. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Unlike fluorine, chlorine can form. Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The reason that the two chlorine. What Bonds With Chlorine.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent What Bonds With Chlorine Two chlorine atoms will each share one electron to get a full outer shell and form a stable cl 2. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted to the nucleus of both. The slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom, to. What Bonds With Chlorine.