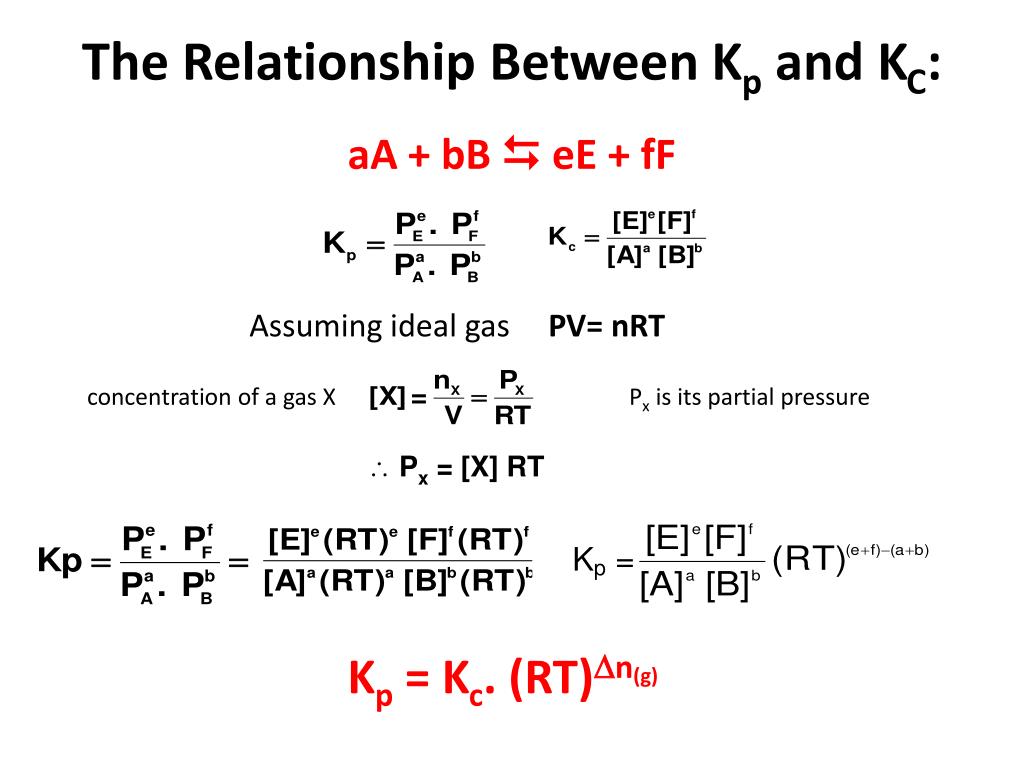
Is Kp And Kc The Same . \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. K p is equilibrium constant used when. However, the difference between the two constants is that \ (k_c\) is defined by molar. Relation between kp and kc. Kp is an equilibrium constant written with. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. For the reaction, 2 so 2 (g) +. K p and k c are the equilibrium constant of an ideal gaseous mixture. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions.
from www.slideserve.com
Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. Kp is an equilibrium constant written with. K p and k c are the equilibrium constant of an ideal gaseous mixture. Relation between kp and kc. However, the difference between the two constants is that \ (k_c\) is defined by molar. K p is equilibrium constant used when. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. For the reaction, 2 so 2 (g) +.
PPT Chemical equilibrium PowerPoint Presentation, free download ID
Is Kp And Kc The Same K p is equilibrium constant used when. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Relation between kp and kc. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. K p and k c are the equilibrium constant of an ideal gaseous mixture. For the reaction, 2 so 2 (g) +. However, the difference between the two constants is that \ (k_c\) is defined by molar. Kp is an equilibrium constant written with. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. K p is equilibrium constant used when.
From byjus.com
6. Why the value of kp and kc depends on the temperature only but not Is Kp And Kc The Same Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. Relation between kp and kc. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. K p is. Is Kp And Kc The Same.
From general.chemistrysteps.com
Kp and Kc Relationship Chemistry Steps Is Kp And Kc The Same K p and k c are the equilibrium constant of an ideal gaseous mixture. Relation between kp and kc. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. K p is equilibrium constant used when. However, the difference between the two constants is. Is Kp And Kc The Same.
From www.youtube.com
Relation between Kc and Kp with one solved example YouTube Is Kp And Kc The Same Kp is an equilibrium constant written with. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Relation between kp and kc. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. K p is equilibrium constant used when. Kp and kc are equilibrium constants of. Is Kp And Kc The Same.
From www.chemistrynotmystery.com
What Is the Relation between Kc and Kp? Chemistry!!! Not Mystery Is Kp And Kc The Same Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation.. Is Kp And Kc The Same.
From www.slideserve.com
PPT Chemical equilibrium PowerPoint Presentation, free download ID Is Kp And Kc The Same Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. K p and k c are the equilibrium constant of an ideal gaseous mixture. K p is equilibrium constant used when. From the equation, k p is equal to k c. Is Kp And Kc The Same.
From www.youtube.com
Equilibria Relationship between equilibrium constants Kp & Kc. YouTube Is Kp And Kc The Same \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. K p and k c are the equilibrium constant of an ideal gaseous mixture. Relation between kp and kc. For the reaction, 2 so 2 (g) +. From. Is Kp And Kc The Same.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership Is Kp And Kc The Same The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. K p and k c are the equilibrium constant of an ideal gaseous mixture. For the reaction, 2 so 2 (g) +. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Understanding the relationship between. Is Kp And Kc The Same.
From chemistry-desk.blogspot.com
Relation Between Kp and Kc │Chemistry Desk Is Kp And Kc The Same Kp is an equilibrium constant written with. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Kc=kp when the mols of gas on. Is Kp And Kc The Same.
From byjus.com
Establish relationship between Kp Kc? Is Kp And Kc The Same Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. K p and k c are the equilibrium constant of an ideal gaseous mixture. From the equation, k p is equal to k c. Is Kp And Kc The Same.
From www.toppr.com
The relationship between Kp and Kc is correctly shown as Is Kp And Kc The Same From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. Relation between kp and kc. For the reaction, 2 so 2 (g) +. The. Is Kp And Kc The Same.
From askfilo.com
Derive the relation between Kp and Kc for the following reactions. Filo Is Kp And Kc The Same Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. However, the difference between the two constants is that \ (k_c\) is defined by molar. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. K p. Is Kp And Kc The Same.
From testbook.com
Relation Between Kp and Kc With Definitions, Derivation, Units Is Kp And Kc The Same K p is equilibrium constant used when. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Kp is an equilibrium constant. Is Kp And Kc The Same.
From www.wizeprep.com
Kc vs Kp Wize University Chemistry Textbook Wizeprep Is Kp And Kc The Same For the reaction, 2 so 2 (g) +. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. From the equation, k p is equal to k c if the same number of moles of gas. Is Kp And Kc The Same.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID1992007 Is Kp And Kc The Same Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. For the reaction, 2 so 2 (g) +. K p and k c are the equilibrium constant of an ideal gaseous mixture. Relation between kp and. Is Kp And Kc The Same.
From www.youtube.com
Relationship between kc kp kx and kn YouTube Is Kp And Kc The Same K p and k c are the equilibrium constant of an ideal gaseous mixture. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Kp is an equilibrium constant written with. From the equation, k p is equal to k c if the same number of moles of. Is Kp And Kc The Same.
From slideplayer.com
Chapter 15 Chemical Equilibrium John A. Schreifels Chemistry ppt download Is Kp And Kc The Same From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. K p and k c are the equilibrium constant of an ideal gaseous mixture.. Is Kp And Kc The Same.
From www.youtube.com
Relationship Between Kp and Kc YouTube Is Kp And Kc The Same From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. K p is equilibrium constant used when. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of. Is Kp And Kc The Same.
From lucabaldwin56.blogspot.com
Relationship Between Kp And Kc Relationship Between Kc, Kp, Kx and Kn Is Kp And Kc The Same For the reaction, 2 so 2 (g) +. However, the difference between the two constants is that \ (k_c\) is defined by molar. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. K p is equilibrium constant used when. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions,. Is Kp And Kc The Same.
From lucabaldwin56.blogspot.com
Relationship Between Kp And Kc Relationship Between Kc, Kp, Kx and Kn Is Kp And Kc The Same Relation between kp and kc. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. For the reaction, 2. Is Kp And Kc The Same.
From www.youtube.com
Relationships between Kp and Kc (Chemical Equilibrium Concept) YouTube Is Kp And Kc The Same K p is equilibrium constant used when. Relation between kp and kc. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. However, the difference between the two. Is Kp And Kc The Same.
From slideplayer.com
Equilibrium. ppt download Is Kp And Kc The Same Kp is an equilibrium constant written with. Relation between kp and kc. However, the difference between the two constants is that \ (k_c\) is defined by molar. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. K p and k c are the. Is Kp And Kc The Same.
From www.bharatagritech.com
What Are Kp And Kc? What Is Relation Between Them? Quora, 59 OFF Is Kp And Kc The Same \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. However, the difference between the two constants is that \ (k_c\) is defined by molar. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. From the equation, k p is equal to k c if the same number of moles of gas are. Is Kp And Kc The Same.
From www.youtube.com
Derivation of Relation between Kp and Kc Class11 Chemistry Ch Is Kp And Kc The Same \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Kp is an equilibrium constant written with. For the reaction, 2 so. Is Kp And Kc The Same.
From www.bharatagritech.com
Learn How The Equilibrium Constants Kc And Kp Are Chemistry, 51 OFF Is Kp And Kc The Same For the reaction, 2 so 2 (g) +. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. However, the difference between the two constants is that \ (k_c\) is. Is Kp And Kc The Same.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2224637 Is Kp And Kc The Same K p is equilibrium constant used when. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Kp is an equilibrium constant. Is Kp And Kc The Same.
From www.youtube.com
Kp= Kc (RT)ΔnWhat is the relation between KP and KC? Is Kp And Kc The Same From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. Kp is an equilibrium constant written with. However, the difference between the two constants. Is Kp And Kc The Same.
From www.vrogue.co
Kp And Kc Relationship Chemistry Steps vrogue.co Is Kp And Kc The Same For the reaction, 2 so 2 (g) +. Relation between kp and kc. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship. Is Kp And Kc The Same.
From www.youtube.com
Relation between Kp and Kc YouTube Is Kp And Kc The Same K p is equilibrium constant used when. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. K p and k c are the equilibrium constant of an ideal gaseous mixture. Relation between kp and kc. Kp is an equilibrium constant written with. The. Is Kp And Kc The Same.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Is Kp And Kc The Same The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. K p is equilibrium constant used when. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. From the equation, k p. Is Kp And Kc The Same.
From byjus.com
Kp and kc relationship below temperature 12.5k Is Kp And Kc The Same Relation between kp and kc. Kp is an equilibrium constant written with. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. K p is equilibrium constant used when. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. K p and. Is Kp And Kc The Same.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Is Kp And Kc The Same However, the difference between the two constants is that \ (k_c\) is defined by molar. From the equation, k p is equal to k c if the same number of moles of gas are on both sides of the chemical equation. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. The equilibrium constant of a. Is Kp And Kc The Same.
From www.vrogue.co
Kp And Kc Relationship Chemistry Steps vrogue.co Is Kp And Kc The Same Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. Kp is an equilibrium constant written with. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. From the equation, k p is equal to k c if the. Is Kp And Kc The Same.
From www.youtube.com
Kp and Kc RELATION CHEMICAL EQUILIBRIUM / INTER FIRST YEAR CHEMISTRY Is Kp And Kc The Same Kp is an equilibrium constant written with. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. However, the difference between the two constants is that \ (k_c\) is defined by molar. Kp and kc are equilibrium constants of ideal gas mixtures considered under reversible reactions. Understanding the. Is Kp And Kc The Same.
From slideplayer.com
Equilibrium Chapter ppt download Is Kp And Kc The Same \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. However, the difference between the two constants is that \ (k_c\) is defined by molar. Kc=kp when the mols of gas on the left side of the equation is equal to the number of mols of gas on the right side of the equation. Understanding the relationship between. Is Kp And Kc The Same.
From www.slideserve.com
PPT Chapter 15 Principles of Chemical Equilibrium PowerPoint Is Kp And Kc The Same Understanding the relationship between k p and k c, the equilibrium constants for gaseous systems and aqueous solutions, respectively, is. However, the difference between the two constants is that \ (k_c\) is defined by molar. Relation between kp and kc. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the. Is Kp And Kc The Same.