What Does An Down Arrow Mean In A Chemical Equation . The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The most common reaction arrow points from left to right. Symbol equations are a quick way of representing chemical reactions. The type of reaction arrow describes the direction in which the chemical reaction proceeds: Many kinds and combinations of arrows can be used. Reaction arrow in a chemical equation. They show us what atoms are involved and how they are bonded together. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Symbol equations always take the. The presence of a double arrow in a chemical equation indicates a reversible reaction. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again.
from www.chegg.com
A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Reaction arrow in a chemical equation. Symbol equations are a quick way of representing chemical reactions. Symbol equations always take the. The most common reaction arrow points from left to right. The presence of a double arrow in a chemical equation indicates a reversible reaction. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. They show us what atoms are involved and how they are bonded together. Many kinds and combinations of arrows can be used.
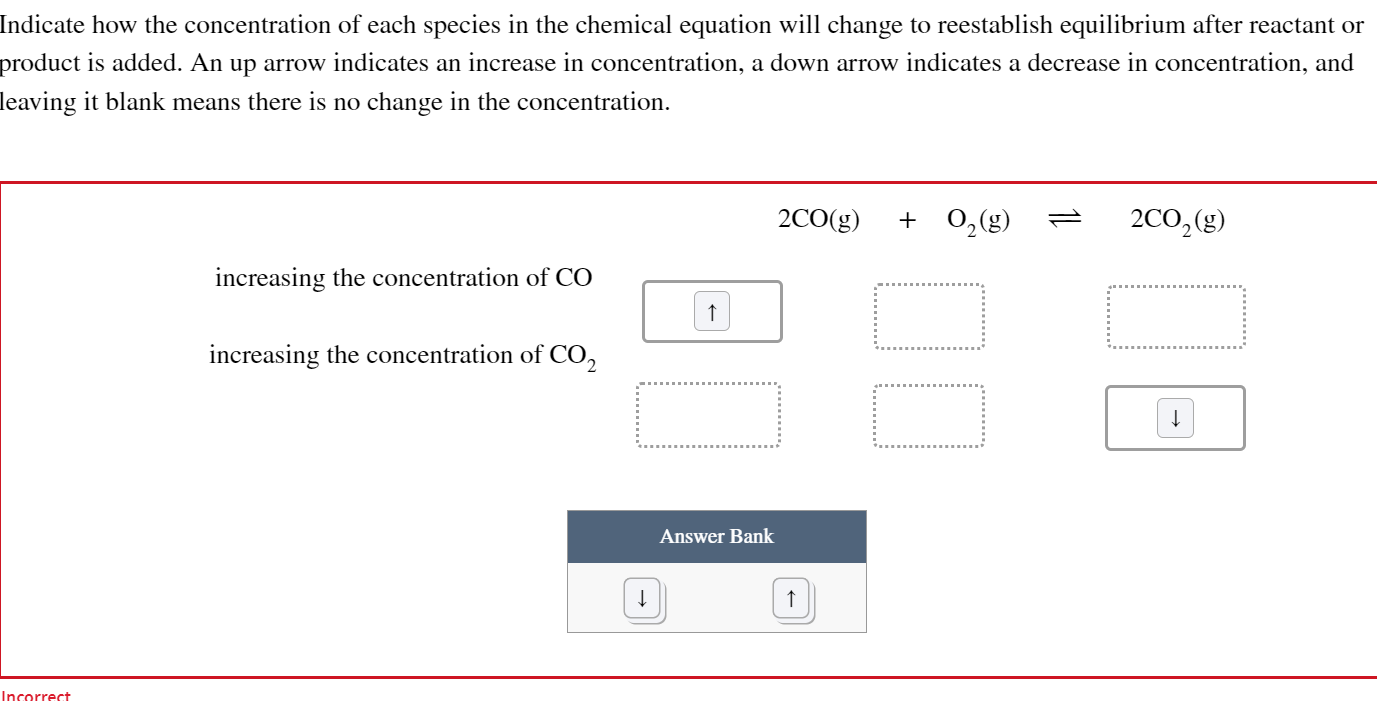
Solved Indicate how the concentration of each species in the
What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. Many kinds and combinations of arrows can be used. Symbol equations are a quick way of representing chemical reactions. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. They show us what atoms are involved and how they are bonded together. Symbol equations always take the. The presence of a double arrow in a chemical equation indicates a reversible reaction. The most common reaction arrow points from left to right. The type of reaction arrow describes the direction in which the chemical reaction proceeds: Reaction arrow in a chemical equation.
From www.masterorganicchemistry.com
The 8 Types of Arrows In Organic Chemistry, Explained Master Organic What Does An Down Arrow Mean In A Chemical Equation Symbol equations always take the. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The presence of a double arrow in a chemical equation indicates a reversible reaction. Reaction arrow in a chemical equation. The most common reaction arrow points from left to right. They show us what atoms are involved. What Does An Down Arrow Mean In A Chemical Equation.
From www.slideserve.com
PPT Ch. 8 Chemical Equations PowerPoint Presentation, free download What Does An Down Arrow Mean In A Chemical Equation The type of reaction arrow describes the direction in which the chemical reaction proceeds: A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. The most common reaction arrow points from left to right. When the reaction is at balance, two arrows with a single spike pointing in opposite. What Does An Down Arrow Mean In A Chemical Equation.
From studylib.net
Chemical equations What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Reaction arrow in a chemical equation. The most common reaction arrow points from left to right. The presence of a double arrow in a chemical equation indicates a reversible reaction. When the reaction is at balance, two arrows with. What Does An Down Arrow Mean In A Chemical Equation.
From www.chemistrysteps.com
Curved Arrows with Practice Problems Chemistry Steps What Does An Down Arrow Mean In A Chemical Equation The type of reaction arrow describes the direction in which the chemical reaction proceeds: Symbol equations always take the. Reaction arrow in a chemical equation. The most common reaction arrow points from left to right. The presence of a double arrow in a chemical equation indicates a reversible reaction. When the reaction is at balance, two arrows with a single. What Does An Down Arrow Mean In A Chemical Equation.
From www.learningchem.info
Resonance and curly arrows What Does An Down Arrow Mean In A Chemical Equation The type of reaction arrow describes the direction in which the chemical reaction proceeds: Symbol equations are a quick way of representing chemical reactions. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. The presence of a double arrow in a chemical equation indicates a reversible reaction. Reaction. What Does An Down Arrow Mean In A Chemical Equation.
From www.youtube.com
Writing chemical equations YouTube What Does An Down Arrow Mean In A Chemical Equation Reaction arrow in a chemical equation. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. Symbol equations always take the. They show. What Does An Down Arrow Mean In A Chemical Equation.
From www.numerade.com
SOLVED For each event stated, indicate how the concentration of each What Does An Down Arrow Mean In A Chemical Equation The most common reaction arrow points from left to right. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. Symbol equations are a quick way of representing chemical reactions. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the. What Does An Down Arrow Mean In A Chemical Equation.
From mavink.com
Chemical Reaction Arrow What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Reaction arrow in a chemical equation. They show us what atoms are involved and how they are bonded together. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The presence. What Does An Down Arrow Mean In A Chemical Equation.
From www.masterorganicchemistry.com
The 8 Types of Arrows In Organic Chemistry, Explained Master Organic What Does An Down Arrow Mean In A Chemical Equation The type of reaction arrow describes the direction in which the chemical reaction proceeds: Reaction arrow in a chemical equation. They show us what atoms are involved and how they are bonded together. Many kinds and combinations of arrows can be used. Symbol equations are a quick way of representing chemical reactions. Symbol equations always take the. The most common. What Does An Down Arrow Mean In A Chemical Equation.
From www.slideserve.com
PPT Chemical Equations and Reactions PowerPoint Presentation, free What Does An Down Arrow Mean In A Chemical Equation The presence of a double arrow in a chemical equation indicates a reversible reaction. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. The most common reaction arrow points from left to right. Symbol equations are a quick way of representing chemical reactions.. What Does An Down Arrow Mean In A Chemical Equation.
From www.chegg.com
Solved Indicate how the concentration of each species in the What Does An Down Arrow Mean In A Chemical Equation Many kinds and combinations of arrows can be used. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The most common reaction arrow points from left to right. The presence of a double arrow in a chemical equation indicates a reversible reaction. They show us what atoms are involved and how. What Does An Down Arrow Mean In A Chemical Equation.
From www.compoundchem.com
Compound Interest General Chemistry What Does An Down Arrow Mean In A Chemical Equation Many kinds and combinations of arrows can be used. Symbol equations are a quick way of representing chemical reactions. The presence of a double arrow in a chemical equation indicates a reversible reaction. The most common reaction arrow points from left to right. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show. What Does An Down Arrow Mean In A Chemical Equation.
From www.youtube.com
Types of Arrows in Organic Chemistry YouTube What Does An Down Arrow Mean In A Chemical Equation The type of reaction arrow describes the direction in which the chemical reaction proceeds: The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Symbol equations are a quick way of. What Does An Down Arrow Mean In A Chemical Equation.
From keslerscience.com
BALANCING CHEMICAL EQUATIONS LESSON PLAN A COMPLETE SCIENCE LESSON What Does An Down Arrow Mean In A Chemical Equation When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. The most common reaction arrow points from left to right. Many kinds and combinations of arrows can be used. Symbol equations always take the. A chemical equation shows the starting compound(s)—the reactants—on the left. What Does An Down Arrow Mean In A Chemical Equation.
From www.expii.com
What Is a Chemical Reaction? — Overview & Examples Expii What Does An Down Arrow Mean In A Chemical Equation The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The type of reaction arrow describes the direction in which the chemical reaction proceeds: Symbol equations always take the. Symbol equations are a quick way of representing chemical reactions. The presence of a double arrow in a chemical equation indicates a reversible. What Does An Down Arrow Mean In A Chemical Equation.
From pamela-has-mayer.blogspot.com
Identify the Appropriate Arrow in the Equation Shown. PamelahasMayer What Does An Down Arrow Mean In A Chemical Equation Reaction arrow in a chemical equation. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The presence of a double arrow in a chemical equation indicates a reversible reaction. The type of reaction arrow describes the direction in which the chemical reaction proceeds: They show us what atoms are involved and. What Does An Down Arrow Mean In A Chemical Equation.
From www.youtube.com
Arrows in Chemistry Different type of Arrows used in chemistry 3D What Does An Down Arrow Mean In A Chemical Equation The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. Symbol equations are a quick way of representing chemical reactions. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. The presence of a double arrow. What Does An Down Arrow Mean In A Chemical Equation.
From www.bartleby.com
Answered Indicate how the concentration of each… bartleby What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. They show us what atoms are involved and how they are bonded together. The most common reaction arrow points from left to right. Many kinds and combinations of arrows can be used. The type of reaction arrow describes the. What Does An Down Arrow Mean In A Chemical Equation.
From www.youtube.com
3 ways to type chemical reaction, chemical equation, reaction arrows in What Does An Down Arrow Mean In A Chemical Equation The presence of a double arrow in a chemical equation indicates a reversible reaction. The most common reaction arrow points from left to right. Many kinds and combinations of arrows can be used. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. When the reaction is at balance,. What Does An Down Arrow Mean In A Chemical Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Equilibrium PowerPoint Presentation, free What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Many kinds and combinations of arrows can be used. Symbol equations are a quick way of representing chemical reactions. Symbol equations always take the. Reaction arrow in a chemical equation. The type of reaction arrow describes the direction in. What Does An Down Arrow Mean In A Chemical Equation.
From www.learningchem.info
Resonance and curly arrows What Does An Down Arrow Mean In A Chemical Equation Reaction arrow in a chemical equation. The most common reaction arrow points from left to right. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products.. What Does An Down Arrow Mean In A Chemical Equation.
From mavink.com
Chemical Reaction Arrow What Does An Down Arrow Mean In A Chemical Equation Symbol equations are a quick way of representing chemical reactions. Reaction arrow in a chemical equation. The most common reaction arrow points from left to right. The type of reaction arrow describes the direction in which the chemical reaction proceeds: Symbol equations always take the. Many kinds and combinations of arrows can be used. They show us what atoms are. What Does An Down Arrow Mean In A Chemical Equation.
From www.slideserve.com
PPT Introduction to Chemical Reactions PowerPoint Presentation, free What Does An Down Arrow Mean In A Chemical Equation Symbol equations always take the. Reaction arrow in a chemical equation. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. The presence of a double arrow in a chemical equation indicates a reversible reaction. The symbol → means the reaction proceeds in a. What Does An Down Arrow Mean In A Chemical Equation.
From www.chegg.com
Solved For each event, indicate how the concentration of What Does An Down Arrow Mean In A Chemical Equation The presence of a double arrow in a chemical equation indicates a reversible reaction. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The most common reaction arrow points from left to right. Reaction arrow in a chemical equation. Many kinds and combinations of arrows can be used. Symbol equations are. What Does An Down Arrow Mean In A Chemical Equation.
From openpress.usask.ca
5.2. OvertheArrow Notation in Chemical Reactions Introduction to What Does An Down Arrow Mean In A Chemical Equation The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. Symbol equations always take the. The most common reaction arrow points from left to right. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Symbol equations are a quick way. What Does An Down Arrow Mean In A Chemical Equation.
From openpress.usask.ca
5.2. OvertheArrow Notation in Chemical Reactions Introduction to What Does An Down Arrow Mean In A Chemical Equation The most common reaction arrow points from left to right. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. Symbol equations always take the. The presence of a double arrow in a chemical equation indicates a reversible reaction. The type of reaction arrow describes the direction in which the chemical reaction. What Does An Down Arrow Mean In A Chemical Equation.
From www.pinterest.com
Conventions for Writing Chemical Equations including Arrows, Phases What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Many kinds and combinations of arrows can be used. They show us what atoms are involved and how they are bonded together. Symbol equations always take the. The presence of a double arrow in a chemical equation indicates a. What Does An Down Arrow Mean In A Chemical Equation.
From sciencenotes.org
What Is a Chemical Equation? Definition and Examples What Does An Down Arrow Mean In A Chemical Equation The type of reaction arrow describes the direction in which the chemical reaction proceeds: The presence of a double arrow in a chemical equation indicates a reversible reaction. Symbol equations are a quick way of representing chemical reactions. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. They. What Does An Down Arrow Mean In A Chemical Equation.
From wordwall.net
Chemical Reactions Concepts and Vocab Quiz What Does An Down Arrow Mean In A Chemical Equation Reaction arrow in a chemical equation. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. The most common reaction arrow points from left to right. Symbol equations are a quick way of representing chemical reactions. The presence of a double arrow in a chemical equation indicates a reversible reaction. Many kinds. What Does An Down Arrow Mean In A Chemical Equation.
From www.youtube.com
Arrow Pushing in Organic Chemistry YouTube What Does An Down Arrow Mean In A Chemical Equation The most common reaction arrow points from left to right. Symbol equations always take the. Many kinds and combinations of arrows can be used. The type of reaction arrow describes the direction in which the chemical reaction proceeds: Reaction arrow in a chemical equation. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the. What Does An Down Arrow Mean In A Chemical Equation.
From www.nagwa.com
Question Video Understanding the Types of Reaction Arrows Nagwa What Does An Down Arrow Mean In A Chemical Equation A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. Reaction arrow in a chemical equation. Symbol equations always take the. Symbol equations are a quick way of representing chemical reactions. Many kinds and combinations of arrows can be used. When the reaction is at balance, two arrows with. What Does An Down Arrow Mean In A Chemical Equation.
From www.chemistryworld.com
Explainer why are curly aka curved arrows used in organic What Does An Down Arrow Mean In A Chemical Equation Reaction arrow in a chemical equation. They show us what atoms are involved and how they are bonded together. The most common reaction arrow points from left to right. When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. A chemical equation shows the. What Does An Down Arrow Mean In A Chemical Equation.
From blog.cambridgecoaching.com
Guide to Deciphering Chemistry Arrows What Does An Down Arrow Mean In A Chemical Equation Many kinds and combinations of arrows can be used. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. The symbol → means the reaction proceeds in a net forward direction, where reactants react and yield products. Symbol equations always take the. When the reaction is at balance, two. What Does An Down Arrow Mean In A Chemical Equation.
From aracely-has-hughes.blogspot.com
What Does the Arrow Mean in a Chemical Equation AracelyhasHughes What Does An Down Arrow Mean In A Chemical Equation Symbol equations are a quick way of representing chemical reactions. The type of reaction arrow describes the direction in which the chemical reaction proceeds: When the reaction is at balance, two arrows with a single spike pointing in opposite directions show that the reaction can be stopped and started again. Reaction arrow in a chemical equation. Symbol equations always take. What Does An Down Arrow Mean In A Chemical Equation.
From www.edrawmax.com
Chemical Symbols and Meanings EdrawMax Online What Does An Down Arrow Mean In A Chemical Equation The most common reaction arrow points from left to right. The type of reaction arrow describes the direction in which the chemical reaction proceeds: Symbol equations always take the. Many kinds and combinations of arrows can be used. They show us what atoms are involved and how they are bonded together. Reaction arrow in a chemical equation. The presence of. What Does An Down Arrow Mean In A Chemical Equation.