Chlorine And Bromine Are Metamerically Electron . Bromine is less reactive, means it reactive more slowly,. Values for electronegativity run from 0 to 4. As you know, both chlorine and bromine are located in group 17 of the periodic. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Electron configuration chart of all elements is mentioned in the table below. The bromine atom has one more electron shell than the chlorine atom. The shorthand electron configuration (or noble gas configuration) as well as. This makes the radius (the distance from the nucleus to the outer shell) of. So, why does that happen? By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. It has 17 protons in the nucleus. Chlorine does have a higher electron affinity than bromine.
from www.numerade.com
The shorthand electron configuration (or noble gas configuration) as well as. Chlorine does have a higher electron affinity than bromine. Bromine is less reactive, means it reactive more slowly,. Values for electronegativity run from 0 to 4. Electron configuration chart of all elements is mentioned in the table below. As you know, both chlorine and bromine are located in group 17 of the periodic. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. So, why does that happen? Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. It has 17 protons in the nucleus.
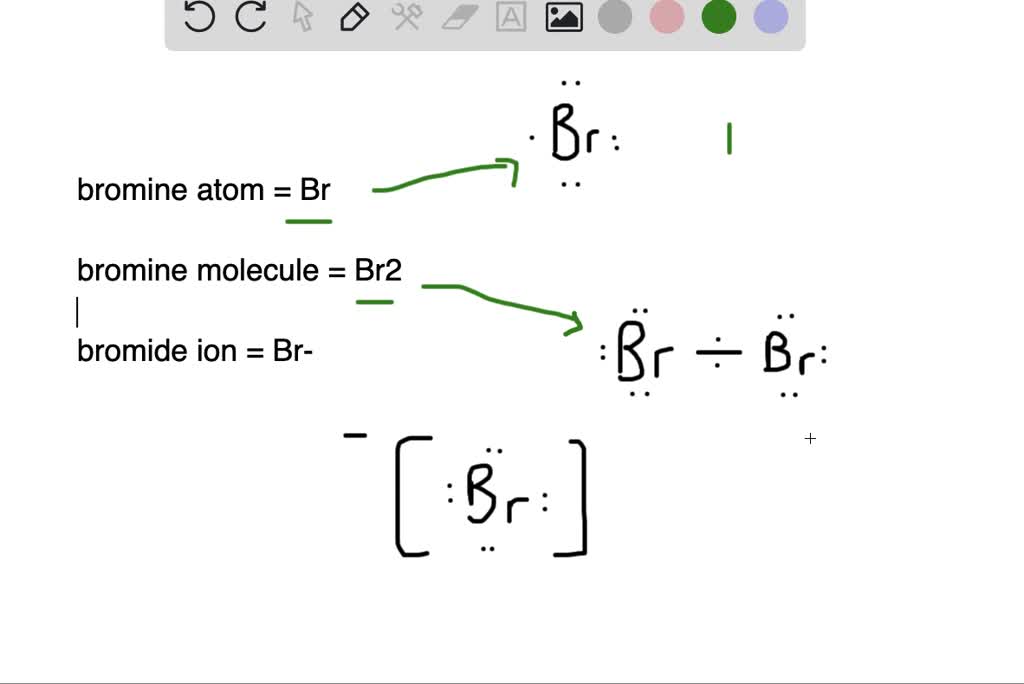
What is the difference between (a) a bromine atom, (b) a bromine
Chlorine And Bromine Are Metamerically Electron Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. It has 17 protons in the nucleus. Values for electronegativity run from 0 to 4. Electron configuration chart of all elements is mentioned in the table below. Bromine is less reactive, means it reactive more slowly,. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The shorthand electron configuration (or noble gas configuration) as well as. So, why does that happen? Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Chlorine does have a higher electron affinity than bromine. This makes the radius (the distance from the nucleus to the outer shell) of. As you know, both chlorine and bromine are located in group 17 of the periodic. The bromine atom has one more electron shell than the chlorine atom.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine And Bromine Are Metamerically Electron So, why does that happen? The bromine atom has one more electron shell than the chlorine atom. Values for electronegativity run from 0 to 4. Chlorine does have a higher electron affinity than bromine. As you know, both chlorine and bromine are located in group 17 of the periodic. It has 17 protons in the nucleus. The relative lower reactivity. Chlorine And Bromine Are Metamerically Electron.
From dokumen.tips
(PDF) Single Covalent Bonds · Draw electron dot structures for each Chlorine And Bromine Are Metamerically Electron It has 17 protons in the nucleus. Bromine is less reactive, means it reactive more slowly,. Values for electronegativity run from 0 to 4. Electron configuration chart of all elements is mentioned in the table below. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Electronegativity is a. Chlorine And Bromine Are Metamerically Electron.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Chlorine And Bromine Are Metamerically Electron As you know, both chlorine and bromine are located in group 17 of the periodic. Bromine is less reactive, means it reactive more slowly,. Values for electronegativity run from 0 to 4. Electron configuration chart of all elements is mentioned in the table below. So, why does that happen? It has 17 protons in the nucleus. Chlorine does have a. Chlorine And Bromine Are Metamerically Electron.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Chlorine And Bromine Are Metamerically Electron By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Chlorine does have a higher electron affinity than bromine. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself.. Chlorine And Bromine Are Metamerically Electron.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Chlorine And Bromine Are Metamerically Electron This makes the radius (the distance from the nucleus to the outer shell) of. The bromine atom has one more electron shell than the chlorine atom. It has 17 protons in the nucleus. Electron configuration chart of all elements is mentioned in the table below. As you know, both chlorine and bromine are located in group 17 of the periodic.. Chlorine And Bromine Are Metamerically Electron.
From jkd-fotografie.blogspot.com
Chlorine Pentafluoride Lewis Dot Diagram First draw the lewis dot Chlorine And Bromine Are Metamerically Electron Electron configuration chart of all elements is mentioned in the table below. This makes the radius (the distance from the nucleus to the outer shell) of. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. As you know, both chlorine and bromine are located in group 17 of the periodic. It has 17 protons in the. Chlorine And Bromine Are Metamerically Electron.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine And Bromine Are Metamerically Electron The bromine atom has one more electron shell than the chlorine atom. It has 17 protons in the nucleus. Values for electronegativity run from 0 to 4. The shorthand electron configuration (or noble gas configuration) as well as. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Electron configuration chart of all elements is mentioned in. Chlorine And Bromine Are Metamerically Electron.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine And Bromine Are Metamerically Electron This makes the radius (the distance from the nucleus to the outer shell) of. Values for electronegativity run from 0 to 4. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the. Chlorine And Bromine Are Metamerically Electron.
From www.numerade.com
SOLVED 'This image shows............... This image shows points 37 1 Chlorine And Bromine Are Metamerically Electron Bromine is less reactive, means it reactive more slowly,. So, why does that happen? Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The bromine atom has one more electron shell than the chlorine atom. As you know, both chlorine and bromine are located in group 17 of the periodic. Electron configuration. Chlorine And Bromine Are Metamerically Electron.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Are Metamerically Electron It has 17 protons in the nucleus. Values for electronegativity run from 0 to 4. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Electron configuration chart of all elements is mentioned in the table below. As you know, both chlorine and bromine are located in group 17. Chlorine And Bromine Are Metamerically Electron.
From manualfixthanedom77.z22.web.core.windows.net
Sodium Oxide Lewis Diagram Chlorine And Bromine Are Metamerically Electron The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine is less reactive, means it reactive more slowly,. This makes the radius (the distance from the nucleus to the outer shell) of. As you know, both chlorine and bromine are located in group 17 of the periodic. The shorthand electron configuration (or noble gas configuration) as. Chlorine And Bromine Are Metamerically Electron.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Chlorine And Bromine Are Metamerically Electron Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The bromine atom has one more electron shell than the chlorine atom. It has 17 protons in the nucleus. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Electron configuration chart. Chlorine And Bromine Are Metamerically Electron.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog Chlorine And Bromine Are Metamerically Electron Bromine is less reactive, means it reactive more slowly,. As you know, both chlorine and bromine are located in group 17 of the periodic. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. This makes the radius (the distance from the nucleus to the outer shell) of. By contrast, chlorine has the. Chlorine And Bromine Are Metamerically Electron.
From www.craiyon.com
Illustration depicting halogens chlorine, bromine, iodine, fluorine Chlorine And Bromine Are Metamerically Electron The relative lower reactivity of bromine makes it exhibits a much greater selectivity. As you know, both chlorine and bromine are located in group 17 of the periodic. It has 17 protons in the nucleus. This makes the radius (the distance from the nucleus to the outer shell) of. Electronegativity is a chemical property which describes how well an atom. Chlorine And Bromine Are Metamerically Electron.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Chlorine And Bromine Are Metamerically Electron Chlorine does have a higher electron affinity than bromine. Values for electronegativity run from 0 to 4. So, why does that happen? Electron configuration chart of all elements is mentioned in the table below. As you know, both chlorine and bromine are located in group 17 of the periodic. Electronegativity is a chemical property which describes how well an atom. Chlorine And Bromine Are Metamerically Electron.
From chemistry291.blogspot.com
Magnesium Bromide FormulaFormula for Magnesium Bromide Chlorine And Bromine Are Metamerically Electron Bromine is less reactive, means it reactive more slowly,. Electron configuration chart of all elements is mentioned in the table below. Values for electronegativity run from 0 to 4. It has 17 protons in the nucleus. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Chlorine does have a higher electron affinity. Chlorine And Bromine Are Metamerically Electron.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Chlorine And Bromine Are Metamerically Electron Electron configuration chart of all elements is mentioned in the table below. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. It has 17 protons in the nucleus. Values for electronegativity run from 0 to 4. Bromine is less reactive, means it reactive more slowly,. By contrast, chlorine has the electronic structure. Chlorine And Bromine Are Metamerically Electron.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine And Bromine Are Metamerically Electron The bromine atom has one more electron shell than the chlorine atom. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. As you know, both chlorine and bromine are located in group 17 of the periodic. It has 17 protons in the nucleus. The relative lower. Chlorine And Bromine Are Metamerically Electron.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Chlorine And Bromine Are Metamerically Electron The bromine atom has one more electron shell than the chlorine atom. It has 17 protons in the nucleus. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. The shorthand electron configuration (or noble gas configuration) as well as. Values for electronegativity run from 0 to 4. Bromine. Chlorine And Bromine Are Metamerically Electron.
From ar.inspiredpencil.com
Electron Configuration Of Chlorine Chlorine And Bromine Are Metamerically Electron Values for electronegativity run from 0 to 4. The shorthand electron configuration (or noble gas configuration) as well as. This makes the radius (the distance from the nucleus to the outer shell) of. It has 17 protons in the nucleus. As you know, both chlorine and bromine are located in group 17 of the periodic. So, why does that happen?. Chlorine And Bromine Are Metamerically Electron.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Chlorine And Bromine Are Metamerically Electron Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Electron configuration chart of all elements is mentioned in the table below. So, why does that happen? This makes the radius (the distance from the nucleus to the outer shell) of. Chlorine does have a higher electron affinity than bromine. The relative lower. Chlorine And Bromine Are Metamerically Electron.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Chlorine And Bromine Are Metamerically Electron Electron configuration chart of all elements is mentioned in the table below. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. So, why does that happen? It has 17 protons in the nucleus. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2. Chlorine And Bromine Are Metamerically Electron.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Chlorine And Bromine Are Metamerically Electron It has 17 protons in the nucleus. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Electron configuration chart of all elements is mentioned in the table below. As you know, both chlorine and bromine are located in group 17 of the periodic. This makes the radius (the distance from the nucleus to the outer shell). Chlorine And Bromine Are Metamerically Electron.
From brainly.com
Which particle represents the size of the bromide ion compared to the Chlorine And Bromine Are Metamerically Electron The bromine atom has one more electron shell than the chlorine atom. So, why does that happen? Values for electronegativity run from 0 to 4. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Chlorine does have a higher electron affinity than bromine. By contrast, chlorine has the electronic structure 1s 2. Chlorine And Bromine Are Metamerically Electron.
From brainly.com
This is a model of a Chlorine atom. How likely is it that this atom Chlorine And Bromine Are Metamerically Electron It has 17 protons in the nucleus. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. The bromine. Chlorine And Bromine Are Metamerically Electron.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Chlorine And Bromine Are Metamerically Electron Chlorine does have a higher electron affinity than bromine. Electron configuration chart of all elements is mentioned in the table below. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. So, why does that happen? By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1.. Chlorine And Bromine Are Metamerically Electron.
From www.linstitute.net
IB DP Chemistry SL复习笔记9.1.10 Electrolytic Cells翰林国际教育 Chlorine And Bromine Are Metamerically Electron Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. It has 17 protons in the nucleus. Chlorine does have a higher electron affinity than bromine. This makes the radius (the distance from the nucleus to the outer shell) of. The relative lower reactivity of bromine makes it exhibits a much greater selectivity.. Chlorine And Bromine Are Metamerically Electron.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Chlorine And Bromine Are Metamerically Electron By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Chlorine does have a higher electron affinity than bromine. The shorthand electron configuration (or noble gas configuration) as well as. So, why does that happen? Electron configuration chart of all elements is mentioned in the table below. It has. Chlorine And Bromine Are Metamerically Electron.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine And Bromine Are Metamerically Electron Bromine is less reactive, means it reactive more slowly,. Chlorine does have a higher electron affinity than bromine. The shorthand electron configuration (or noble gas configuration) as well as. As you know, both chlorine and bromine are located in group 17 of the periodic. It has 17 protons in the nucleus. The relative lower reactivity of bromine makes it exhibits. Chlorine And Bromine Are Metamerically Electron.
From www.youtube.com
The electron gain enthalpy (in \( \mathrm{kJ} / \mathrm{mol} \) ) of Chlorine And Bromine Are Metamerically Electron Chlorine does have a higher electron affinity than bromine. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. It has 17 protons in the nucleus. Bromine is less reactive, means it reactive more slowly,. So, why does that happen? Electron configuration chart of all elements is mentioned in the table below. The. Chlorine And Bromine Are Metamerically Electron.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Chlorine And Bromine Are Metamerically Electron Values for electronegativity run from 0 to 4. This makes the radius (the distance from the nucleus to the outer shell) of. As you know, both chlorine and bromine are located in group 17 of the periodic. The bromine atom has one more electron shell than the chlorine atom. Bromine is less reactive, means it reactive more slowly,. So, why. Chlorine And Bromine Are Metamerically Electron.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chlorine And Bromine Are Metamerically Electron The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. This makes the radius (the distance from the nucleus. Chlorine And Bromine Are Metamerically Electron.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine And Bromine Are Metamerically Electron This makes the radius (the distance from the nucleus to the outer shell) of. The bromine atom has one more electron shell than the chlorine atom. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1.. Chlorine And Bromine Are Metamerically Electron.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Chlorine And Bromine Are Metamerically Electron The bromine atom has one more electron shell than the chlorine atom. Chlorine does have a higher electron affinity than bromine. Values for electronegativity run from 0 to 4. It has 17 protons in the nucleus. The shorthand electron configuration (or noble gas configuration) as well as. Bromine is less reactive, means it reactive more slowly,. So, why does that. Chlorine And Bromine Are Metamerically Electron.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog Chlorine And Bromine Are Metamerically Electron Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. By contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. Chlorine does have a higher electron affinity than bromine. The bromine atom has one more electron shell than the chlorine atom. This. Chlorine And Bromine Are Metamerically Electron.