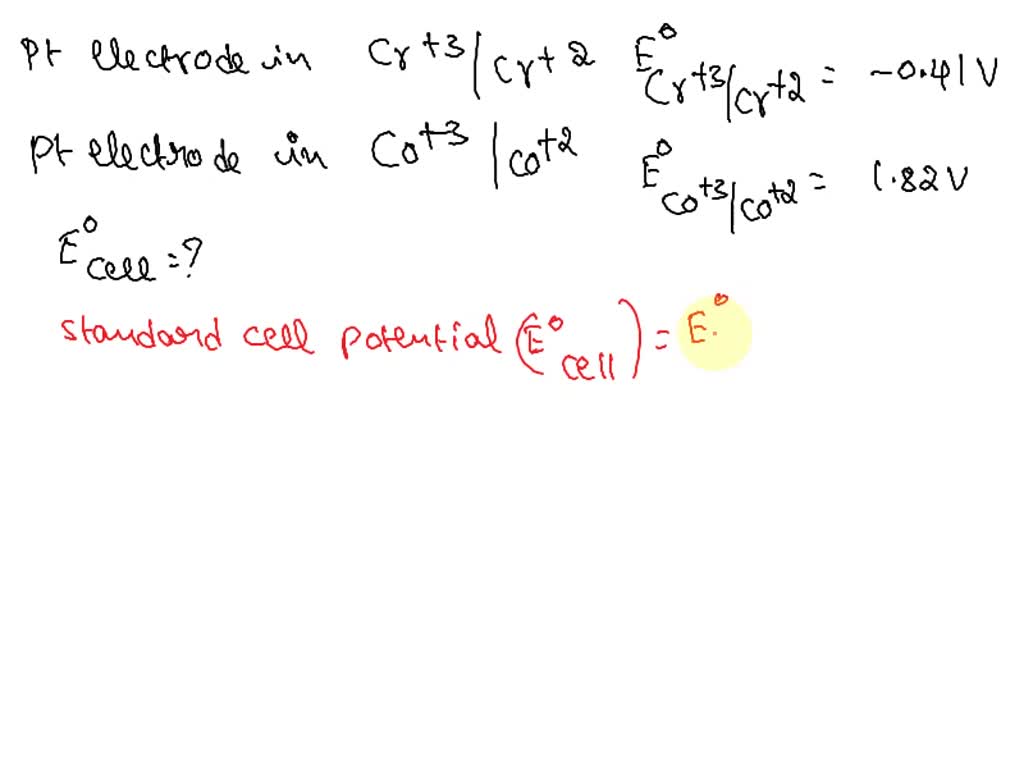Platinum Electrode In Galvanic Cell . One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. Therefore, if one side only has aqueous solutions (and.
from www.numerade.com
One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. Therefore, if one side only has aqueous solutions (and.
SOLVED A galvanic (voltaic) cell consists of an inert platinum
Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic cell consisting of two beakers.
From 2012books.lardbucket.org
Describing Electrochemical Cells Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side only has aqueous solutions (and. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From www.researchgate.net
Structure of the (A) platinum microelectrode and (B) experimental setup Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From solvedlib.com
Galvanic (voltaic) cell consists of an inert platinum… SolvedLib Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From mungfali.com
Galvanic Cell Diagram Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Platinum Electrode In Galvanic Cell A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From studylib.net
A galvanic cell is made from two half cells. platinum electrode is Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin. Platinum Electrode In Galvanic Cell.
From mmerevise.co.uk
Electrochemical Cells MME Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic cell consisting of two beakers. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From www.numerade.com
SOLVED A galvanic (voltaic) cell consists of an inert platinum Platinum Electrode In Galvanic Cell A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.
From www.chegg.com
Solved A galvanic (voltaic) cell consists of an inert Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.
From studylibrenegado.z4.web.core.windows.net
Inert Electrode Galvanic Cell Platinum Electrode In Galvanic Cell A chemist has constructed a galvanic cell consisting of two beakers. Therefore, if one side only has aqueous solutions (and. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From wps.prenhall.com
Media Portfolio Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From question.pandai.org
Standard Electrode Potential Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic cell consisting of two beakers. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Platinum Electrode In Galvanic Cell A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin. Platinum Electrode In Galvanic Cell.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From www.numerade.com
SOLVED3. A galvanic cell consists of an inert platinum electrode in 1. Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic cell consisting of two beakers. Therefore, if one side only has aqueous solutions (and. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.
From www.slideserve.com
PPT The End is in Site! PowerPoint Presentation, free download ID Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.
From www.numerade.com
SOLVED A galvanic (voltaic) cell consists of an electrode composed of Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From www.youtube.com
Galvanic cell or Voltaic cell Definition ,Construction and Working Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side. Platinum Electrode In Galvanic Cell.
From www.numerade.com
SOLVED A galvanic (voltaic) cell consists of an inert platinum Platinum Electrode In Galvanic Cell Therefore, if one side only has aqueous solutions (and. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin. Platinum Electrode In Galvanic Cell.
From www.numerade.com
SOLVED galvanic (voltaic) cell consists of an inert platinum electrode Platinum Electrode In Galvanic Cell You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side only has aqueous solutions (and. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each. Platinum Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Platinum Electrode In Galvanic Cell Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released electrons to the external circuit. You need to have one conductive metal on each side of the galvanic cell. One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. A chemist has constructed a galvanic. Platinum Electrode In Galvanic Cell.
From www.chegg.com
Solved A galvanic (voltaic) cell consists of an inert Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. Therefore, if one side only has aqueous solutions (and. You need to have one conductive metal on each side of the galvanic cell. A chemist has constructed a galvanic cell consisting of two beakers. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.
From www.numerade.com
SOLVED(Short) A nickel electrode is placed in a 1.0 M solution of Platinum Electrode In Galvanic Cell One beaker contains a strip of tin immersed in aqueous sulfuric acid,. A chemist has constructed a galvanic cell consisting of two beakers. You need to have one conductive metal on each side of the galvanic cell. Therefore, if one side only has aqueous solutions (and. Reaction \(\ref{5}\) occurs at the surface of the platinum wire, which conducts the released. Platinum Electrode In Galvanic Cell.