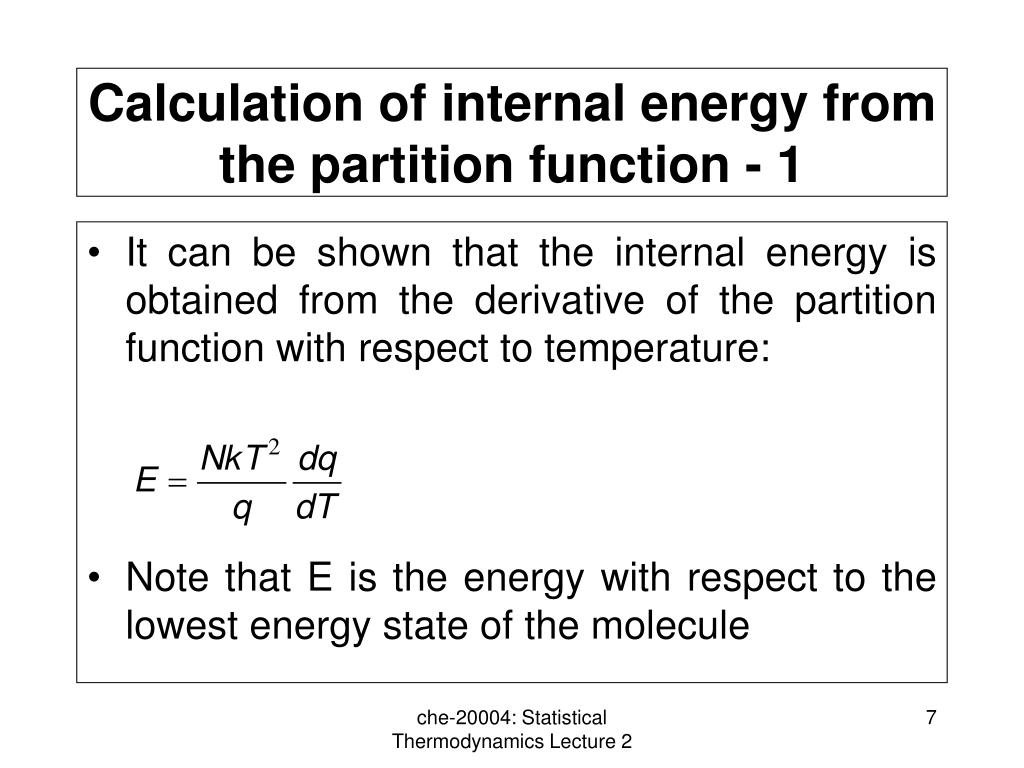
Internal Energy Calculation . Heat and work are both. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. once the temperature increase has occurred, it is impossible to tell whether it was caused by heat or work. describe the work done by a system, heat transfer between objects, and internal energy change of a system. — the internal energy of a system is energy associated with the motion of molecules, atoms, and the particles. — the internal energy can be either potential energy or kinetic energy. — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. the question we're gonna answer is, for this process, what is delta u? Make a list of information about the system including. internal energy formula is the heat energy stocked in gas. how to calculate the internal energy of a thermodynamic system. If a certain amount of heat is applied to gas, the result is that the. We discussed the concepts of work and. The heat flow is equal to the change in the internal energy of. — internal energy is a state function because the internal energy of a system depends upon the state of the.
from www.slideserve.com
— the internal energy of a system is energy associated with the motion of molecules, atoms, and the particles. The heat flow is equal to the change in the internal energy of. internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. how to calculate the internal energy of a thermodynamic system. So, what the change in internal energy for our system?. internal energy is a state function of a system and is an extensive quantity. — the formula for internal energy is: how to calculate internal energy. — the internal energy can be either potential energy or kinetic energy.
PPT CHE20004 PHYSICAL CHEMISTRY STATISTICAL THERMODYNAMICS LECTURE
Internal Energy Calculation it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. Heat and work are both. how to calculate internal energy. — the sum of all the different kinds of energy which the molecules of a substance can possess is called the. — the internal energy (u) of a system is a thermodynamic state function defined as: One can have a corresponding intensive. internal energy formula is the heat energy stocked in gas. internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. When a material is heated or cooled, two changes may happen to the particles within the material: Make a list of information about the system including. describe the work done by a system, heat transfer between objects, and internal energy change of a system. internal energy is a state function of a system and is an extensive quantity. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. the question we're gonna answer is, for this process, what is delta u? If a certain amount of heat is applied to gas, the result is that the.
From eduinput.com
What is Internal EnergyDefinition And Example Internal Energy Calculation the question we're gonna answer is, for this process, what is delta u? — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. So, what the change in internal energy for our system?. internal energy is a state function of a system and is an extensive. Internal Energy Calculation.
From studylib.net
Load and Energy Calculations Department of Mechanical Internal Energy Calculation When a material is heated or cooled, two changes may happen to the particles within the material: once the temperature increase has occurred, it is impossible to tell whether it was caused by heat or work. — the internal energy of a system is energy associated with the motion of molecules, atoms, and the particles. internal energy. Internal Energy Calculation.
From www.youtube.com
5 INTERNAL ENERGY CALCULATION THERMODYNAMICS IIT ADVANCED JEE Internal Energy Calculation it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. — the formula for internal energy is: We discussed the concepts of work and. So, what the change. Internal Energy Calculation.
From www.tessshebaylo.com
What Is The Equation To Solve For Amount Of Heat Energy Tessshebaylo Internal Energy Calculation Δu = the total change in internal energy of a system. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. One can have a corresponding intensive. — the sum of all the different kinds of energy which the molecules of a substance can possess is called the. . Internal Energy Calculation.
From www.youtube.com
Internal Energy GCSE Physics Revision YouTube Internal Energy Calculation the question we're gonna answer is, for this process, what is delta u? internal energy is a state function of a system and is an extensive quantity. internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. So, what the change in internal energy for our system?. . Internal Energy Calculation.
From ar.inspiredpencil.com
Total Energy Formula Physics Internal Energy Calculation internal energy formula is the heat energy stocked in gas. — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. Δu = the total change in internal energy of a system. how to calculate the internal energy of a thermodynamic system. revision notes on 16.1.1. Internal Energy Calculation.
From www.tec-science.com
Calculation of the internal energy for ideal gases tecscience Internal Energy Calculation — the internal energy (u) of a system is a thermodynamic state function defined as: — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. — the sum of all the different kinds of energy which the molecules of a substance can possess is called the.. Internal Energy Calculation.
From testbook.com
Internal Energy Formula Learn its Concept, Formula and Examples Internal Energy Calculation — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. The heat flow is equal to the change in the internal energy of. — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. revision notes on 16.1.1. Internal Energy Calculation.
From www.youtube.com
Changes in Enthalpy and Internal Energy (Example) YouTube Internal Energy Calculation Δu = the total change in internal energy of a system. it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. — the internal energy of a system is energy associated with the motion of molecules, atoms, and the particles. The heat flow is equal to the change. Internal Energy Calculation.
From www.youtube.com
Calculating changes in internal energy from equations of state YouTube Internal Energy Calculation internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a. Internal Energy Calculation.
From thechemistrynotes.com
Energy Diagram Internal Energy Calculation Δu = the total change in internal energy of a system. So, what the change in internal energy for our system?. — internal energy is a state function because the internal energy of a system depends upon the state of the. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure. Internal Energy Calculation.
From www.youtube.com
Ideal Adiabatic Process of A Piston Expanding (Find Work and Total Internal Energy Calculation it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. revision notes on 16.1.1 internal energy for the cie a level physics syllabus, written by the physics experts at save my exams. So, what the change in internal energy for our system?. — the formula for internal. Internal Energy Calculation.
From byjus.com
The internal energy of an ideal gas is sum of energy of Internal Energy Calculation When a material is heated or cooled, two changes may happen to the particles within the material: Δu = the total change in internal energy of a system. once the temperature increase has occurred, it is impossible to tell whether it was caused by heat or work. — this review focuses on internal energy and enthalpy of nonelectrolyte. Internal Energy Calculation.
From www.youtube.com
Thermodynamics 13 Determination of Internal energy calculation Internal Energy Calculation Δu = the total change in internal energy of a system. — the internal energy of a system is energy associated with the motion of molecules, atoms, and the particles. If a certain amount of heat is applied to gas, the result is that the. internal energy increases when the temperature rises and states or phases transition from. Internal Energy Calculation.
From www.filscihub.com
[CHEM/PHYS MODULE] GUIDE for Calculating Internal Energy (First Law of Internal Energy Calculation — internal energy is a state function because the internal energy of a system depends upon the state of the. — the internal energy can be either potential energy or kinetic energy. When a material is heated or cooled, two changes may happen to the particles within the material: — this review focuses on internal energy and. Internal Energy Calculation.
From adpokat.github.io
What Is Adiabatic Process Internal Energy Calculation — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. So, what the change in internal energy for our system?. calculate the work, heat transfer, and internal energy change in a simple process. describe the work done by a system, heat transfer between objects, and internal. Internal Energy Calculation.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Internal Energy Calculation describe the work done by a system, heat transfer between objects, and internal energy change of a system. how to calculate the internal energy of a thermodynamic system. Δu = the total change in internal energy of a system. — the sum of all the different kinds of energy which the molecules of a substance can possess. Internal Energy Calculation.
From dxocskfly.blob.core.windows.net
Energy And Power Equation at James Erdmann blog Internal Energy Calculation So, what the change in internal energy for our system?. internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. — the sum of all the different kinds of energy which the molecules of a substance can possess is called the. it may be difficult to calculate the. Internal Energy Calculation.
From www.researchgate.net
Conversion of energy and internal energy Download Scientific Internal Energy Calculation — the sum of all the different kinds of energy which the molecules of a substance can possess is called the. We discussed the concepts of work and. Δu = the total change in internal energy of a system. the change in the internal energy of a system is the sum of the heat transferred and the work. Internal Energy Calculation.
From www.grc.nasa.gov
Enthalpy Internal Energy Calculation describe the work done by a system, heat transfer between objects, and internal energy change of a system. — internal energy is a state function because the internal energy of a system depends upon the state of the. how to calculate the internal energy of a thermodynamic system. When a material is heated or cooled, two changes. Internal Energy Calculation.
From dokumen.tips
(PDF) TDC (MAJOR) COURSE First Year · energy, heat, work, internal Internal Energy Calculation If a certain amount of heat is applied to gas, the result is that the. describe the work done by a system, heat transfer between objects, and internal energy change of a system. So, what the change in internal energy for our system?. — internal energy is a state function because the internal energy of a system depends. Internal Energy Calculation.
From pediaa.com
Difference Between Enthalpy and Internal Energy Definition, Units Internal Energy Calculation So, what the change in internal energy for our system?. The main difference between enthalpy and. once the temperature increase has occurred, it is impossible to tell whether it was caused by heat or work. — the formula for internal energy is: Make a list of information about the system including. The heat flow is equal to the. Internal Energy Calculation.
From eduinput.com
What is Internal EnergyDefinition And Example Internal Energy Calculation We discussed the concepts of work and. Make a list of information about the system including. once the temperature increase has occurred, it is impossible to tell whether it was caused by heat or work. So, what the change in internal energy for our system?. Heat and work are both. the question we're gonna answer is, for this. Internal Energy Calculation.
From www.toppr.com
Calculate y (ratio of Cp and Cv ) for triatomic linear gas at high Internal Energy Calculation One can have a corresponding intensive. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. When a material is heated or cooled, two changes may happen to the particles within the material: — the internal energy (u) of a system is a thermodynamic state function defined as: . Internal Energy Calculation.
From physics.stackexchange.com
thermodynamics Calculating work from enthaply and internal energy Internal Energy Calculation it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. We discussed the concepts of work and. — the sum of all the different kinds of energy which the molecules of a substance can possess is called the. once the temperature increase has occurred, it is impossible. Internal Energy Calculation.
From app.jove.com
Internal Energy Concept Physics JoVe Internal Energy Calculation We discussed the concepts of work and. — the formula for internal energy is: — the internal energy (u) of a system is a thermodynamic state function defined as: The heat flow is equal to the change in the internal energy of. — internal energy is a state function because the internal energy of a system depends. Internal Energy Calculation.
From www.researchgate.net
(PDF) Exact Calculation of the Internal Energy of the Ideal Gas in Internal Energy Calculation internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. the change in the internal energy of a system is the sum of the heat transferred and the work done. One can have a corresponding intensive. Heat and work are both. calculate the work, heat transfer, and internal. Internal Energy Calculation.
From www.tec-science.com
Calculation of the internal energy for ideal gases tecscience Internal Energy Calculation — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. internal energy formula is the heat energy stocked in gas. Make a list of information about the system including. the question we're gonna answer is, for this process, what is delta u? — the change in internal. Internal Energy Calculation.
From www.youtube.com
Calculating Internal Energy and Fluid Temperature Using the NFEE YouTube Internal Energy Calculation how to calculate internal energy. We discussed the concepts of work and. revision notes on 16.1.1 internal energy for the cie a level physics syllabus, written by the physics experts at save my exams. it may be difficult to calculate the total internal energy, depending on which forms of energy you take into account. The main difference. Internal Energy Calculation.
From www.coursehero.com
[Solved] Question. QUESTION 2 1. A rigid tank contains a hot fluid that Internal Energy Calculation internal energy formula is the heat energy stocked in gas. how to calculate internal energy. revision notes on 16.1.1 internal energy for the cie a level physics syllabus, written by the physics experts at save my exams. When a material is heated or cooled, two changes may happen to the particles within the material: describe the. Internal Energy Calculation.
From www.researchgate.net
Free energy calculation of a) Water internal angle from a Deep model Internal Energy Calculation the change in the internal energy of a system is the sum of the heat transferred and the work done. So, what the change in internal energy for our system?. If a certain amount of heat is applied to gas, the result is that the. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours. Internal Energy Calculation.
From www.slidemake.com
Einstein Theory Of Specific Heat Presentation Internal Energy Calculation internal energy is a state function of a system and is an extensive quantity. — this review focuses on internal energy and enthalpy of nonelectrolyte liquids, vapours and gases, either pure or mixed. Heat and work are both. calculate the work, heat transfer, and internal energy change in a simple process. When a material is heated or. Internal Energy Calculation.
From www.researchgate.net
(a−c) Internal energy calculation as a function of the applied electric Internal Energy Calculation internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. internal energy is a state function of a system and is an extensive quantity. — the sum of all the different kinds of energy which the molecules of a substance can possess is called the. revision notes. Internal Energy Calculation.
From www.slideserve.com
PPT CHE20004 PHYSICAL CHEMISTRY STATISTICAL THERMODYNAMICS LECTURE Internal Energy Calculation Make a list of information about the system including. The main difference between enthalpy and. internal energy increases when the temperature rises and states or phases transition from solid to liquid and liquid to. Heat and work are both. — the sum of all the different kinds of energy which the molecules of a substance can possess is. Internal Energy Calculation.
From www.teachertube.com
example calculation of internal energy Internal Energy Calculation — the change in internal energy of a system is the sum of \(w\) and \(q\), which is a state function. The main difference between enthalpy and. describe the work done by a system, heat transfer between objects, and internal energy change of a system. We discussed the concepts of work and. internal energy is a state. Internal Energy Calculation.