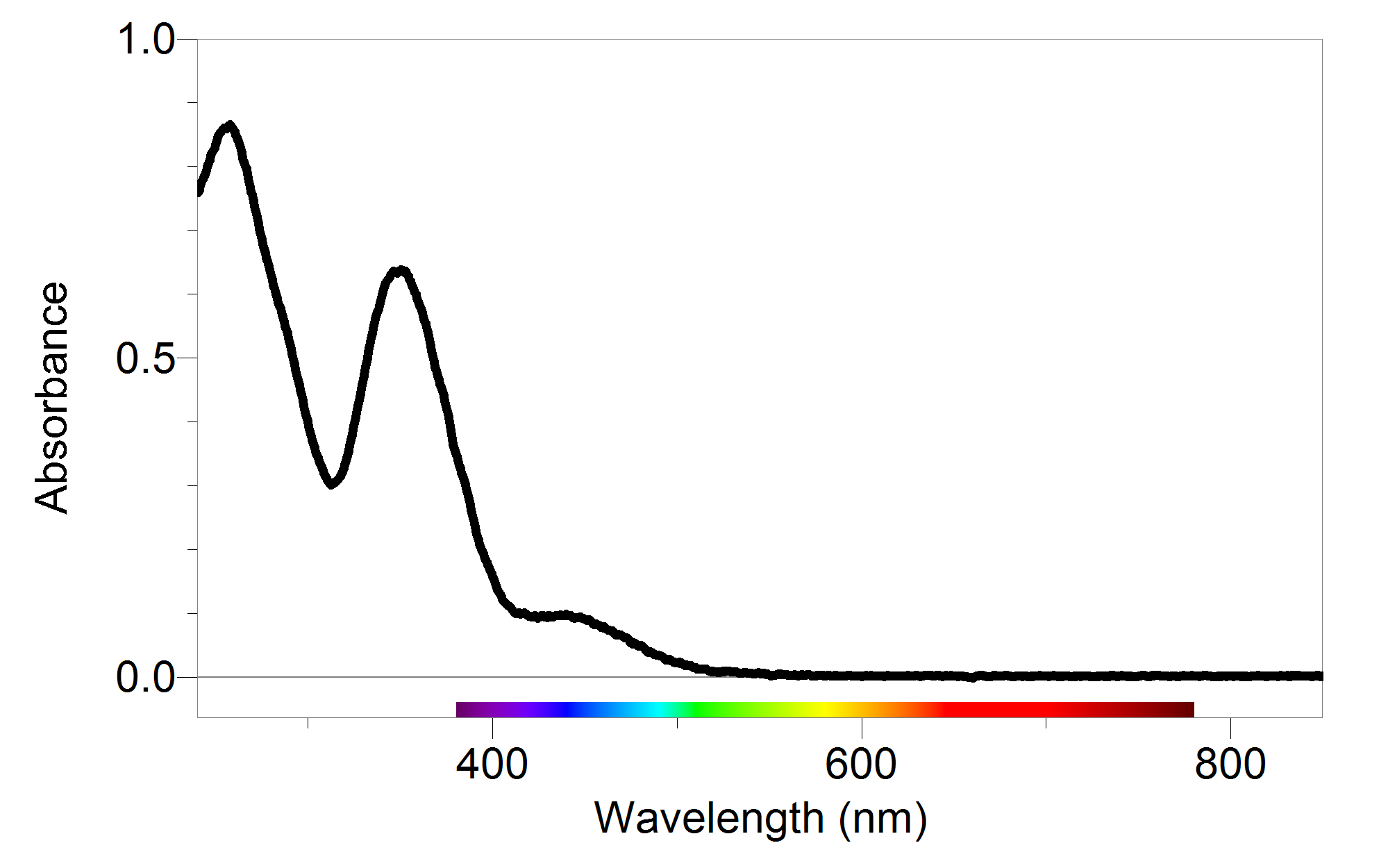
How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width . Here is an example showing how to use beer’s law. Suppose a small amount of stray radiation (p s ) always leaked into your. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. For a single wavelength, a is absorbance (unitless, usually seen as arb.
from www.vernier.com
A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Here is an example showing how to use beer’s law. Suppose a small amount of stray radiation (p s ) always leaked into your. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. For a single wavelength, a is absorbance (unitless, usually seen as arb.
Decoding Your Absorbance Readings Vernier
How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. For a single wavelength, a is absorbance (unitless, usually seen as arb. Suppose a small amount of stray radiation (p s ) always leaked into your. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. Here is an example showing how to use beer’s law.
From www.researchgate.net
Absorbance data as a function of time during photodegradation as How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Suppose a small amount of stray radiation (p s ) always leaked into your. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. Here is an example. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Spectroscopic data. ( A, B ) Folded fraction (derived from the How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Suppose a small amount of stray radiation (p s ) always leaked into your. Here is an example showing how to use beer’s law. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From absorb--00.blogspot.com
3 INFO E ABSORBANCE WITH VIDEO TUTORIAL * Absord How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. For a single wavelength, a is absorbance (unitless, usually seen as arb. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Concentration vs. absorbance curve generated from data collected using How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width For a single wavelength, a is absorbance (unitless, usually seen as arb. Here is an example showing how to use beer’s law. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample,. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Representative change in absorbance data for the addition of Fe(NTA) to How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width For a single wavelength, a is absorbance (unitless, usually seen as arb. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Suppose a small amount of stray. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From airekacells.com
UVvis Spectrophotometer Cuvette Selection Guide How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width For a single wavelength, a is absorbance (unitless, usually seen as arb. Suppose a small amount of stray radiation (p s ) always leaked into your. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. The absorbance of the sample is used with the equation for the standard curve to calculate. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.bartleby.com
Answered Table II Cuvette Concentration… bartleby How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Suppose a small amount of stray radiation (p s ) always leaked into your. Here is an example showing how to use beer’s law. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. For a single wavelength, a is absorbance (unitless, usually seen as. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From openwetware.org
UserJavier Vinals Camallonga/Notebook/Javier Vinals notebook/2013/09 How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.infosem.org
What Are The Benefits Of Using Quartz Cuvettes For Spectroscopic How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of.. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Absorbance Spectrum of undoped and B doped Cu2O Thin Films Download How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. For a single wavelength, a is absorbance (unitless, usually seen as arb. Here is an example showing how to use beer’s law. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.coursehero.com
[Solved] May someone show how to plot the given data of absorbance vs How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. For a single wavelength, a is absorbance (unitless, usually seen as arb. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Absorbance measures the amount of light. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.spectroscopyonline.com
Valuable Techniques for Repeatable Absorbance Measurements How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Suppose a small amount of stray radiation (p s ) always leaked into your. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Here is an example showing how to use beer’s law. Absorbance measures the amount of light with a specific wavelength that a. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Relative absorbance (∆A) measurement with the incremental (a) and How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. Here is an example showing how to use beer’s law. For a single wavelength, a is absorbance (unitless, usually seen as arb. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. Suppose a. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.vernier.com
Decoding Your Absorbance Readings Vernier How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From mavink.com
Absorbance Vs. Concentration Graph How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. The absorbance of the sample is used with the equation for. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.chemedx.org
A Quick Comparison of Experimental Results Chemical Education Xchange How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Suppose a small amount of stray radiation (p s ) always leaked into your. For a single wavelength, a is absorbance (unitless, usually seen as arb. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Here is an example showing how to use beer’s law.. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Example for the analysis of absorbance data from the sedimentation How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Suppose a small amount of stray radiation (p s ) always leaked into your. For a single wavelength, a is absorbance (unitless, usually seen as arb. Here is an example showing how to use beer’s law. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. In absorbance spectroscopy (also known as. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.chegg.com
Solved Question 2 (1 point) Absorbance values for five How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Here is an example showing how to use beer’s law. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. For a single wavelength, a is absorbance (unitless, usually seen as arb. The absorbance of the sample is used with the equation for the standard curve. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.chegg.com
Solved PART II Absorbance vs. Wavelength of Solution C Fill How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Suppose a small amount of stray radiation (p s ) always leaked into your. The absorbance of the. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.numerade.com
SOLVED 7 ( 20 pts The acid/base indicator HIn undergoes the following How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. For a single wavelength, a is absorbance (unitless, usually seen as arb. Suppose a small amount of. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.chegg.com
Solved QUESTION 4 Given the absorbance and concentration How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Here is an example showing how to use beer’s law. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. A spectrophotometer in an instrument that measures the. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Calibration curve of absorbance versus concentration. Download How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Here is an example showing how to use beer’s law. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. The absorbance of the sample is used with. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Absorbance of the PRESAGE TM samples as a function of dose for three How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. Here is an example showing how to use beer’s law. A spectrophotometer in an instrument that measures the amount of light absorbed at a. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From chart-studio.plotly.com
Absorbance Vs. Time (Without Inhibitor) line chart made by Lutzjake How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width For a single wavelength, a is absorbance (unitless, usually seen as arb. Here is an example showing how to use beer’s law. Suppose a small amount of stray radiation (p s ) always leaked into your. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. A spectrophotometer in an instrument that. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From definitionhjo.blogspot.com
Definition Of Absorbance In Chemistry DEFINITION HJO How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Suppose a small amount of stray radiation (p s ) always leaked into your. Here is an example showing how to use beer’s law. For a single wavelength, a is absorbance (unitless, usually seen as arb. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. A spectrophotometer in an instrument that. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From airekacells.com
UVvis Spectrophotometer Cuvette Selection Guide How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. Here is an example showing how to use beer’s law. For a single wavelength, a is absorbance (unitless, usually seen as arb. Absorbance measures the amount of light with a specific wavelength that a given. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Dependence of reaction velocity upon enzyme concentration. Assays were How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. The absorbance of the sample is used with the equation for the standard curve to calculate the. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.numerade.com
SOLVED NOTE The absorbance data used here ai for illustrative How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Here is an example showing how to use beer’s law. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. The. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From rheolution.com
Understanding Absorbance at Specific Wavelengths How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. For a single wavelength, a is absorbance (unitless, usually seen as arb. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
Relative absorption spectra (normalized data with the absorbance value How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. Suppose a small amount of stray radiation (p s ) always leaked into your. Absorbance measures the. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.chegg.com
Solved Question 32 A student measures the absorbance of a How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
The relationship between absorbance measured on a spectrophotometer How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. Here is an example showing how to use beer’s law. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration. Suppose a small amount of stray radiation (p s ) always leaked into your.. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.chegg.com
Absorbance data were collected at various How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be used to. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. For a single wavelength, a is absorbance (unitless, usually seen as. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width For a single wavelength, a is absorbance (unitless, usually seen as arb. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample as a function of. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. Here is an example showing how. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.
From www.researchgate.net
The absorbance curve of TiO 2 layers Download Scientific Diagram How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width For a single wavelength, a is absorbance (unitless, usually seen as arb. Here is an example showing how to use beer’s law. Absorbance measures the amount of light with a specific wavelength that a given substance prevents from passing through. In absorbance spectroscopy (also known as absorption spectroscopy), an optical spectrometer measures the amount of light absorbed by a sample. How Would The Absorbance Data Be Different If You Used Cuvettes That Were Twice The Width.