Acetic Acid Titration . in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. 10 analysis of acids and bases by titration purpose. It enables the concentration of the ch3cooh to be determined from the. Initially the ph is due. the incremental process of adding the ch3cooh solution is called titration.
from wizedu.com
a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. 10 analysis of acids and bases by titration purpose. Initially the ph is due.
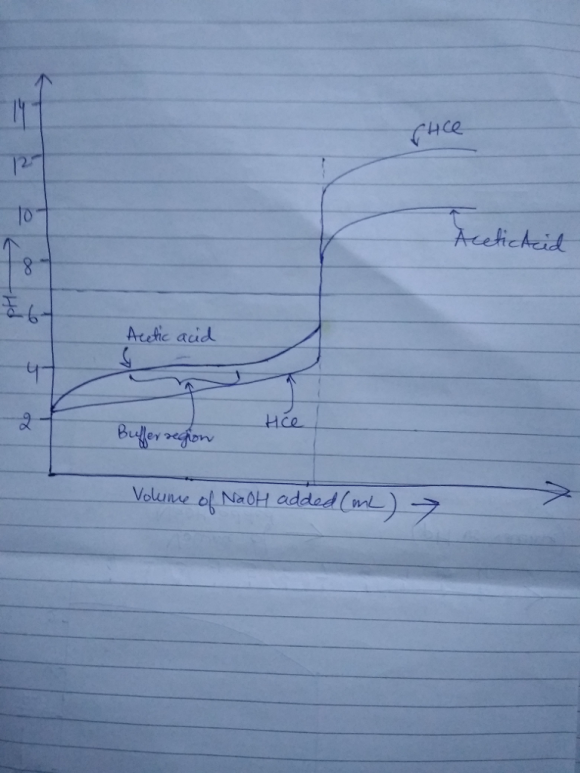
3. Plot a schematic titration curve for acetic acid with NaOH
Acetic Acid Titration in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. 10 analysis of acids and bases by titration purpose. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. the incremental process of adding the ch3cooh solution is called titration. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. Initially the ph is due. It enables the concentration of the ch3cooh to be determined from the.
From studylib.net
The Titration of Acetic Acid in Vinegar Acetic Acid Titration the incremental process of adding the ch3cooh solution is called titration. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. It enables the concentration of the ch3cooh to be determined from the. 10 analysis of acids. Acetic Acid Titration.
From chempedia.info
Acetic Acid titration curve Big Chemical Encyclopedia Acetic Acid Titration Initially the ph is due. 10 analysis of acids and bases by titration purpose. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in. Acetic Acid Titration.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Acetic Acid Titration in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. Initially the ph is due. 10 analysis of acids and bases by titration purpose. titration of acetic acid with. Acetic Acid Titration.
From www.scribd.com
THE TITRATION OF ACETIC ACID IN VINEGAR.docx Titration Analytical Acetic Acid Titration 10 analysis of acids and bases by titration purpose. Initially the ph is due. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. the incremental process of adding the ch3cooh solution is called titration. It enables. Acetic Acid Titration.
From mavink.com
Titration Curve Of Acetic Acid Acetic Acid Titration Initially the ph is due. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. It enables the concentration of the ch3cooh to be determined from the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is a technique often used to find the. Acetic Acid Titration.
From wizedu.com
3. Plot a schematic titration curve for acetic acid with NaOH Acetic Acid Titration It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. Initially the ph is due.. Acetic Acid Titration.
From chempedia.info
Acetic Acid titration curve Big Chemical Encyclopedia Acetic Acid Titration in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh). Acetic Acid Titration.
From mungfali.com
Titration Curve Of Acetic Acid Acetic Acid Titration titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. the incremental process of adding the ch3cooh solution is called titration. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. 10 analysis of acids and bases by titration purpose. Initially the ph is due. It enables. Acetic Acid Titration.
From chempedia.info
Acetic Acid titration curve Big Chemical Encyclopedia Acetic Acid Titration a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. 10 analysis of acids and bases by titration purpose. Initially the ph is due. the incremental process of adding the ch3cooh solution is called titration. titration. Acetic Acid Titration.
From www.researchgate.net
Conductometric titration of acetic acid in the mixture (branches B and Acetic Acid Titration the incremental process of adding the ch3cooh solution is called titration. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. in this experiment, a technique known as a titration will be used to determine the concentration. Acetic Acid Titration.
From studylib.net
Titration of acetic Acid 101 Acetic Acid Titration the incremental process of adding the ch3cooh solution is called titration. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. It enables the concentration of the ch3cooh to be determined from the. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. a titration is a. Acetic Acid Titration.
From www.youtube.com
ACETIC ACID TITRATION BY SODIUM HYDROXIDE YouTube Acetic Acid Titration Initially the ph is due. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. It enables the concentration of. Acetic Acid Titration.
From www.youtube.com
Titration Acetic Acid with NaOH YouTube Acetic Acid Titration 10 analysis of acids and bases by titration purpose. It enables the concentration of the ch3cooh to be determined from the. Initially the ph is due. the incremental process of adding the ch3cooh solution is called titration. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in this experiment, acetic acid (ch 3 cooh). Acetic Acid Titration.
From mavink.com
Titration Of Acetic Acid With Naoh Acetic Acid Titration in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. 10 analysis of acids and bases by titration purpose. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is a technique often used to find the concentration of a solute in a solution,. Acetic Acid Titration.
From www.researchgate.net
Titration curves for acetic acid, monochloroacetic acid and Acetic Acid Titration in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. It enables the concentration of the ch3cooh to be. Acetic Acid Titration.
From mungfali.com
Titration Curve Of Acetic Acid Acetic Acid Titration the incremental process of adding the ch3cooh solution is called titration. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. Initially the ph is due. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. 10 analysis of acids and bases by titration purpose. a. Acetic Acid Titration.
From www.researchgate.net
Conductometric titration of acetic acid in the mixture (branches B and Acetic Acid Titration 10 analysis of acids and bases by titration purpose. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. in this experiment, acetic acid (ch. Acetic Acid Titration.
From mavink.com
Titration Curve Of Acetic Acid Acetic Acid Titration 10 analysis of acids and bases by titration purpose. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. the incremental process of adding the ch3cooh solution is called titration. It enables the concentration of the ch3cooh to be determined from the. in this experiment, a technique known. Acetic Acid Titration.
From www.chegg.com
Solved Determination of Acetic Acid Concentration in Vinegar Acetic Acid Titration in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. It enables the concentration of the ch3cooh to be determined from the. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as. Acetic Acid Titration.
From feqtudy.blogspot.com
Indicator For Titration Of Acetic Acid And Sodium Hydroxide FEQTUDY Acetic Acid Titration Initially the ph is due. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. titration of acetic. Acetic Acid Titration.
From www.researchgate.net
Single acid titration curves (nitric acid and acetic acid). Download Acetic Acid Titration the incremental process of adding the ch3cooh solution is called titration. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. 10 analysis of acids and bases by titration purpose. in this experiment, acetic acid (ch. Acetic Acid Titration.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Acetic Acid Titration in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. a titration is a technique often used to find the concentration of a solute in a. Acetic Acid Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Acetic Acid Titration in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. the incremental process of adding the ch3cooh solution is called titration. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. 10 analysis of acids and bases by titration purpose. in this experiment, acetic acid. Acetic Acid Titration.
From mungfali.com
Titration Curve Of Acetic Acid Acetic Acid Titration a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. Initially the ph is due. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. 10 analysis of. Acetic Acid Titration.
From www.researchgate.net
Titration curve of acetic acid at 30 °C (Ve = equivalence volume Acetic Acid Titration Initially the ph is due. It enables the concentration of the ch3cooh to be determined from the. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in this experiment, acetic acid (ch 3 cooh) is the. Acetic Acid Titration.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Acetic Acid Titration in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. 10 analysis of acids and bases by titration purpose. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due. the incremental process of adding the ch3cooh solution is called titration. a. Acetic Acid Titration.
From chempedia.info
Acetic Acid titration curve Big Chemical Encyclopedia Acetic Acid Titration It enables the concentration of the ch3cooh to be determined from the. Initially the ph is due. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. 10 analysis of acids and bases by titration purpose. a titration is a technique often used to find the concentration of. Acetic Acid Titration.
From annie-chemistry.blogspot.com
PreAP Chemistry Blog Acetic Acid in Vinegar Titration lab Acetic Acid Titration It enables the concentration of the ch3cooh to be determined from the. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. 10 analysis of acids and bases by titration purpose. in this experiment, a technique known as a titration will be used to determine the concentration of acetic. Acetic Acid Titration.
From www.youtube.com
Experiment 5 pH Titration of Acetic Acid by Sodium Carbonate Acetic Acid Titration It enables the concentration of the ch3cooh to be determined from the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. in this experiment, acetic. Acetic Acid Titration.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Acetic Acid Titration Initially the ph is due. the incremental process of adding the ch3cooh solution is called titration. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. 10 analysis of acids and bases by titration purpose. a titration is a technique often used to find the concentration of a solute in a solution, though it may. Acetic Acid Titration.
From www.researchgate.net
Titration curves for acetic acid, monochloroacetic acid and Acetic Acid Titration 10 analysis of acids and bases by titration purpose. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in this experiment, a technique known. Acetic Acid Titration.
From www.chegg.com
Solved Titration of Acetic Acid Titration of Acetic Acid in Acetic Acid Titration a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. the incremental process of adding the ch3cooh solution is called titration. in this experiment, acetic. Acetic Acid Titration.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Acetic Acid Titration 10 analysis of acids and bases by titration purpose. the incremental process of adding the ch3cooh solution is called titration. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. a titration is a technique often used to find the concentration of a solute in a solution, though. Acetic Acid Titration.
From www.studypool.com
SOLUTION C Lab 6 Titration Of Acetic Acid In Vinegar Studypool Acetic Acid Titration 10 analysis of acids and bases by titration purpose. in this experiment, acetic acid (ch 3 cooh) is the analyte and sodium hydroxide (naoh) is the standard. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used in other analyses, such as determining the.. Acetic Acid Titration.
From www.researchgate.net
Representative ITC enthalpograms. a Acetic acid titration with NaOH Acetic Acid Titration the incremental process of adding the ch3cooh solution is called titration. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. It enables the concentration of the ch3cooh to be determined from the. a titration is a technique often used to find the concentration of a solute in a solution, though it may also be used. Acetic Acid Titration.