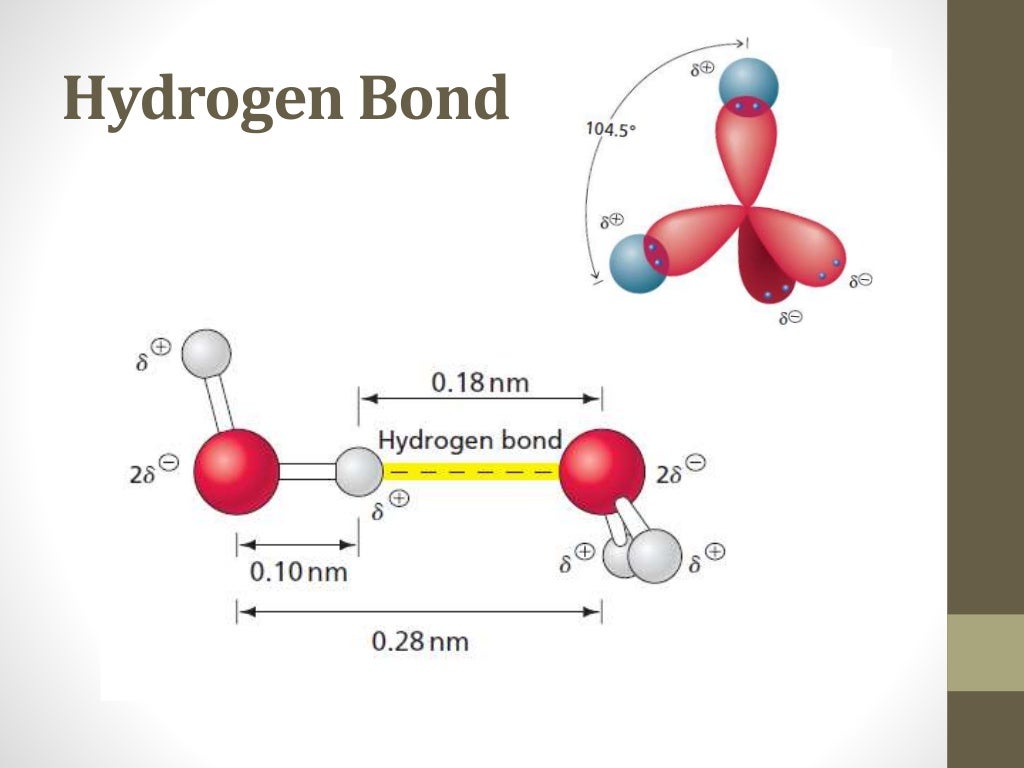
Water Definition In Biochemistry . It is needed by all known forms of life. As an oxide of hydrogen, water is formed when. Although water consists of simple. Building up from microscopic basics to observed complex functions,. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. water (chemical formula: water in biological and chemical processes. Molecules that are ionic or polar dissolve. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water can be split by electrolysis into hydrogen and oxygen. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water, like carbon, has a special role in living things.
from www.slideshare.net
H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. Molecules that are ionic or polar dissolve. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. Building up from microscopic basics to observed complex functions,. water (chemical formula: the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Although water consists of simple. water in biological and chemical processes. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water, like carbon, has a special role in living things.
Biochemistry of Water
Water Definition In Biochemistry water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. As an oxide of hydrogen, water is formed when. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water can be split by electrolysis into hydrogen and oxygen. Although water consists of simple. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. It is needed by all known forms of life. water, like carbon, has a special role in living things. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. Building up from microscopic basics to observed complex functions,. Molecules that are ionic or polar dissolve. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water (chemical formula: water in biological and chemical processes.
From www.slideshare.net
Biochemistry Honors Water Definition In Biochemistry Although water consists of simple. water in biological and chemical processes. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water can be split by electrolysis into hydrogen and oxygen. Molecules that are ionic or polar dissolve. water (chemical formula: Building up from microscopic basics to observed. Water Definition In Biochemistry.
From www.slideserve.com
PPT Water and Biochemistry PowerPoint Presentation, free download Water Definition In Biochemistry Molecules that are ionic or polar dissolve. It is needed by all known forms of life. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to. Water Definition In Biochemistry.
From mavink.com
Diagram Of Water Molecule Water Definition In Biochemistry water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water (chemical formula: As an oxide of hydrogen, water is formed when. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Molecules. Water Definition In Biochemistry.
From farhanborhan.weebly.com
Water Molecule SBK3013 Biochemistry Water Definition In Biochemistry Molecules that are ionic or polar dissolve. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. It is needed by all known forms of. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry water in biological and chemical processes. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water (chemical formula: Although water consists of simple. As an oxide of hydrogen, water is formed when. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to. Water Definition In Biochemistry.
From www.dreamstime.com
Water H2O Molecule Models Blue and Chemical Formulas Natural. Ecology Water Definition In Biochemistry It is needed by all known forms of life. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. Molecules that are ionic or polar dissolve. the polarity of the. Water Definition In Biochemistry.
From www.youtube.com
Biochemistry 3.0 Water and solubility YouTube Water Definition In Biochemistry the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Although water consists of simple. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. It is needed by all known forms of life.. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry It is needed by all known forms of life. water (chemical formula: the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. water. Water Definition In Biochemistry.
From www.youtube.com
Water in BiochemistryProperties of water in biochemistryWater in Water Definition In Biochemistry Although water consists of simple. It is needed by all known forms of life. Building up from microscopic basics to observed complex functions,. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water, like carbon, has a special role in living things. Molecules that are ionic or polar. Water Definition In Biochemistry.
From www.studocu.com
Biochemistry of the Cell Biochemistry of the Cell Properties of Water Water Definition In Biochemistry It is needed by all known forms of life. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. water, like carbon, has a special role in living things. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. Building. Water Definition In Biochemistry.
From www.dreamstime.com
Water molecule stock vector. Illustration of oxygen, life 55633092 Water Definition In Biochemistry As an oxide of hydrogen, water is formed when. water can be split by electrolysis into hydrogen and oxygen. Building up from microscopic basics to observed complex functions,. water in biological and chemical processes. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. the polarity of the. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry It is needed by all known forms of life. water in biological and chemical processes. As an oxide of hydrogen, water is formed when. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to. Water Definition In Biochemistry.
From slideplayer.com
Intro. To Biochemistry Water, Solutions and pH ppt download Water Definition In Biochemistry the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Building up from microscopic basics to observed complex functions,. It is needed by all known forms of life. water can be split by electrolysis into hydrogen and oxygen. water (figure 1.23) is. Water Definition In Biochemistry.
From www.biologyonline.com
Water Definition and Examples Biology Online Dictionary Water Definition In Biochemistry water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water (chemical formula: As an oxide of hydrogen, water is formed when. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. . Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry water, like carbon, has a special role in living things. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water in biological and chemical processes. As an oxide of hydrogen, water is formed when. Building up from microscopic basics to observed complex functions,. water can be. Water Definition In Biochemistry.
From slidetodoc.com
BIOCHEMISTRY 2 2 PROPERTIES OF WATER Polarity Water Water Definition In Biochemistry the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. water, like carbon, has a special role in living things. water in biological and chemical processes. Although water consists of simple. It is needed by all known forms of life. water. Water Definition In Biochemistry.
From www.slideshare.net
Water Biochemistry Water Definition In Biochemistry It is needed by all known forms of life. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. Molecules that are ionic or polar. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Although water consists of simple. water in biological and chemical processes. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. Molecules that are. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry Although water consists of simple. As an oxide of hydrogen, water is formed when. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Molecules that are ionic or polar dissolve. It is needed by all known forms of life. water can be. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that. Water Definition In Biochemistry.
From www.scribd.com
Aspects of Biochemistry Water PDF Properties Of Water Liquids Water Definition In Biochemistry Molecules that are ionic or polar dissolve. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. water, like carbon, has a special role in living things. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry As an oxide of hydrogen, water is formed when. water, like carbon, has a special role in living things. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. water in biological and chemical processes. the polarity of the water molecule and its resulting hydrogen bonding make water. Water Definition In Biochemistry.
From www.slideserve.com
PPT Water and Biochemistry PowerPoint Presentation, free download Water Definition In Biochemistry water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. As an oxide of hydrogen, water is formed when. water in biological and chemical processes. Building up from microscopic basics. Water Definition In Biochemistry.
From www.studocu.com
SBI4U Biochemistry Notes Unit 1 Biochemistry Importance of Water Water Definition In Biochemistry to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water in biological and chemical processes. Molecules that are ionic or polar dissolve. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. As an oxide of hydrogen, water is. Water Definition In Biochemistry.
From www.pinterest.com
illustration of biochemistry, Water molecule consists of two hydrogen Water Definition In Biochemistry water (chemical formula: water, like carbon, has a special role in living things. Although water consists of simple. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. Molecules that are ionic or polar dissolve. As an oxide of hydrogen, water is formed when. water in biological. Water Definition In Biochemistry.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID89333 Water Definition In Biochemistry As an oxide of hydrogen, water is formed when. water (chemical formula: Molecules that are ionic or polar dissolve. water, like carbon, has a special role in living things. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. H 2 o) refers to a chemical substance consisting of. Water Definition In Biochemistry.
From www.scribd.com
Biochemistry Water PDF Water Definition In Biochemistry It is needed by all known forms of life. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. water in biological and chemical processes. Building up from microscopic basics to observed complex functions,. As an oxide of hydrogen, water is formed when.. Water Definition In Biochemistry.
From thebiologs.blogspot.com
The BioLogs CAPE 1 Water Introduction to it's BIOchemistry Water Definition In Biochemistry water can be split by electrolysis into hydrogen and oxygen. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. to understand life,. Water Definition In Biochemistry.
From www.studocu.com
Biochemistry10 N/A 18 Chapter 2 Water Chemical Properties of Water Water Definition In Biochemistry water (chemical formula: water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. water can be split by electrolysis into hydrogen and oxygen. water, like carbon, has a special role in living things. Building up from microscopic basics to observed complex functions,. to understand life, we. Water Definition In Biochemistry.
From www.youtube.com
Biochemistry Water, PH and Buffers Part 1 tutorial YouTube Water Definition In Biochemistry Molecules that are ionic or polar dissolve. As an oxide of hydrogen, water is formed when. the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen. Water Definition In Biochemistry.
From www.slideshare.net
Water Biochemistry Water Definition In Biochemistry water (chemical formula: As an oxide of hydrogen, water is formed when. Although water consists of simple. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. water, like. Water Definition In Biochemistry.
From www.youtube.com
Water Importance and Physical Properties Basic Biochemistry Water Definition In Biochemistry water can be split by electrolysis into hydrogen and oxygen. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. As an oxide of hydrogen, water is formed when. water, like carbon, has a special role in living things. water in biological and chemical processes. H 2. Water Definition In Biochemistry.
From www.youtube.com
Biochemistry pt. 1 Properties of Water YouTube Water Definition In Biochemistry As an oxide of hydrogen, water is formed when. Although water consists of simple. water, like carbon, has a special role in living things. water in biological and chemical processes. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom. to understand life, we begin the discussion with. Water Definition In Biochemistry.
From www.slideshare.net
Biochemistry of Water Water Definition In Biochemistry Molecules that are ionic or polar dissolve. water, like carbon, has a special role in living things. It is needed by all known forms of life. water in biological and chemical processes. water (figure 1.23) is described as a solvent because of its ability to dissolve many, but not all, molecules. to understand life, we begin. Water Definition In Biochemistry.
From www.scribd.com
Water Biochemistry PDF Water Definition In Biochemistry water, like carbon, has a special role in living things. Building up from microscopic basics to observed complex functions,. to understand life, we begin the discussion with the basics of water, because everything that happens in cells,. Molecules that are ionic or polar dissolve. H 2 o) refers to a chemical substance consisting of two hydrogen atoms attached. Water Definition In Biochemistry.