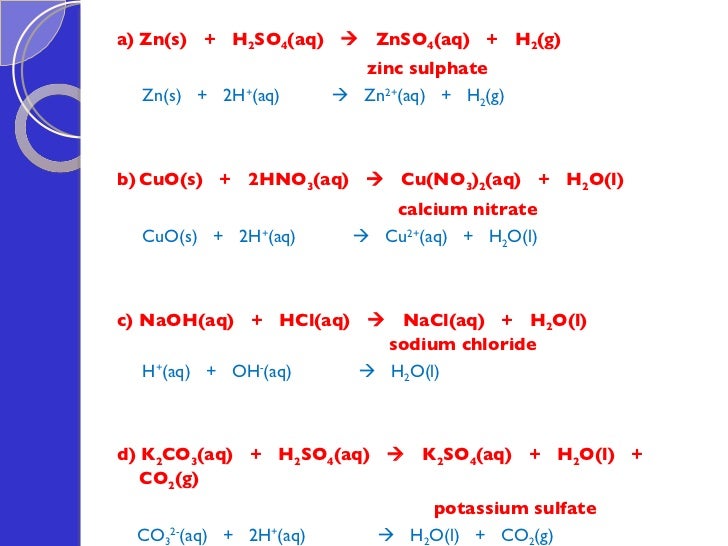
Zinc Sulfide And Hydrochloric Acid . zinc reacts with halogens in the presence of moisture: Zn + cl₂ → zncl₂. this chemistry video tutorial explains how to predict the products of the. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. in this video, we'll be exploring the reaction between zinc metal and.
from www.slideshare.net
zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + cl₂ → zncl₂. this chemistry video tutorial explains how to predict the products of the. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. in this video, we'll be exploring the reaction between zinc metal and. zinc reacts with halogens in the presence of moisture: zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide.
Acids And Bases
Zinc Sulfide And Hydrochloric Acid Zn + cl₂ → zncl₂. in this video, we'll be exploring the reaction between zinc metal and. zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the. zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. Zn + cl₂ → zncl₂. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc Sulfide And Hydrochloric Acid Zn + cl₂ → zncl₂. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zinc reacts with halogens in the presence of moisture: zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide.. Zinc Sulfide And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0223 Zinc Sulfide And Hydrochloric Acid this chemistry video tutorial explains how to predict the products of the. zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. Zn + cl₂ → zncl₂. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l. Zinc Sulfide And Hydrochloric Acid.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Zinc Sulfide And Hydrochloric Acid the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + cl₂ → zncl₂. in this video, we'll be exploring the. Zinc Sulfide And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Sulfide And Hydrochloric Acid zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. Zn + cl₂ → zncl₂. zinc reacts with halogens in the presence of moisture:. Zinc Sulfide And Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Sulfide And Hydrochloric Acid when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. this chemistry video tutorial explains how to predict the products of the. . Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc Sulfide And Hydrochloric Acid the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. zinc reacts with halogens in the presence of moisture: zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. Zn + cl₂ →. Zinc Sulfide And Hydrochloric Acid.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Zinc Sulfide And Hydrochloric Acid this chemistry video tutorial explains how to predict the products of the. Zn + cl₂ → zncl₂. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4. Zinc Sulfide And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Sulfide And Hydrochloric Acid when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the. Zn + cl₂ → zncl₂. zns + 2 hcl → zncl_2 + h_2s. Zinc Sulfide And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Sulfide And Hydrochloric Acid this chemistry video tutorial explains how to predict the products of the. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid):. Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
Zinc and Hydrochloric Acid Reaction chemistry grade10science Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. this chemistry video tutorial explains how to predict the products of the. in this video, we'll be exploring the reaction between zinc metal and.. Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc Sulfide And Hydrochloric Acid in this video, we'll be exploring the reaction between zinc metal and. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. zinc reacts with halogens in the presence of moisture: the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces. Zinc Sulfide And Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Sulfide And Hydrochloric Acid zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. Zn + cl₂ → zncl₂. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. this chemistry video tutorial explains how to predict the. Zinc Sulfide And Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Sulfide And Hydrochloric Acid this chemistry video tutorial explains how to predict the products of the. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. in this video, we'll be exploring the reaction between zinc metal and. zinc reacts with halogens in the presence of moisture:. Zinc Sulfide And Hydrochloric Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Sulfide And Hydrochloric Acid zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. zinc reacts with halogens in the presence of moisture: Zn + cl₂ → zncl₂. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid.. Zinc Sulfide And Hydrochloric Acid.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Zinc Sulfide And Hydrochloric Acid when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): this chemistry video tutorial explains how to predict the products of the. in this video, we'll be exploring the reaction between zinc metal and. zns + 2 hcl → zncl_2 + h_2s zinc sulfide. Zinc Sulfide And Hydrochloric Acid.
From www.numerade.com
SOLVED Write the balanced equations for 1. The reaction of zinc Zinc Sulfide And Hydrochloric Acid when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the. in this video, we'll be exploring the reaction between zinc metal and. . Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
Xth Class, Physical Science, Acids, Bases and SaltsReaction of Zinc Zinc Sulfide And Hydrochloric Acid when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Zn + cl₂ → zncl₂. zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. the reaction of zinc sulphide (z n s) with. Zinc Sulfide And Hydrochloric Acid.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy Zinc Sulfide And Hydrochloric Acid this chemistry video tutorial explains how to predict the products of the. zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. . Zinc Sulfide And Hydrochloric Acid.
From dxopfgxpq.blob.core.windows.net
Zinc And Hydrochloric Acid Reaction Explained at John Crawford blog Zinc Sulfide And Hydrochloric Acid in this video, we'll be exploring the reaction between zinc metal and. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. when. Zinc Sulfide And Hydrochloric Acid.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc Sulfide And Hydrochloric Acid the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. this chemistry video tutorial explains how to predict the products of the. zinc reacts with halogens in the presence of moisture: zinc sulfide can be used to produce hydrogen sulfide in a lab. Zinc Sulfide And Hydrochloric Acid.
From www.numerade.com
SOLVEDWrite a balanced equation for each of the following reactions Zinc Sulfide And Hydrochloric Acid in this video, we'll be exploring the reaction between zinc metal and. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. Zn + cl₂ → zncl₂. this chemistry video tutorial explains how to predict the products of the. when zinc metal is. Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
Single replacement(displacement) reactionreaction of zinc with Zinc Sulfide And Hydrochloric Acid in this video, we'll be exploring the reaction between zinc metal and. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide.. Zinc Sulfide And Hydrochloric Acid.
From www.slideshare.net
Acids And Bases Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: in this video, we'll be exploring the reaction between zinc metal and. the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. Zn + cl₂ → zncl₂. zns + 2 hcl → zncl_2 +. Zinc Sulfide And Hydrochloric Acid.
From spark.adobe.com
Zinc and Hydrochloric Acid Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): this chemistry. Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
How to Balance Zn(OH)2 + HCl = ZnCl2 + H2O (Zinc Hydroxide Zinc Sulfide And Hydrochloric Acid zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen. Zinc Sulfide And Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Sulfide And Hydrochloric Acid Zn + cl₂ → zncl₂. zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the. the reaction of zinc sulphide (z n s) with. Zinc Sulfide And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen chloride to produce zinc chloride and hydrogen sulfide. this chemistry video tutorial explains how to predict the products of the. Zn + cl₂ → zncl₂. when zinc metal is submerged into a quantity of. Zinc Sulfide And Hydrochloric Acid.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper Zinc Sulfide And Hydrochloric Acid the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. this chemistry video tutorial explains how to predict the products of the. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid):. Zinc Sulfide And Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc with dilute hydrochloric acid or sulphuric acid....Zn Zinc Sulfide And Hydrochloric Acid Zn + cl₂ → zncl₂. zinc reacts with halogens in the presence of moisture: the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. this chemistry video tutorial explains how to predict the products of the. in this video, we'll be exploring the. Zinc Sulfide And Hydrochloric Acid.
From www.alamy.com
Labelled diagram for laboratory preparation of hydrogen from zinc and Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): this chemistry video. Zinc Sulfide And Hydrochloric Acid.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: Zn + cl₂ → zncl₂. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric. Zinc Sulfide And Hydrochloric Acid.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc Sulfide And Hydrochloric Acid in this video, we'll be exploring the reaction between zinc metal and. zinc reacts with halogens in the presence of moisture: when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces. Zinc Sulfide And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Zinc Sulfide And Hydrochloric Acid zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like hydrochloric acid. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): zns + 2 hcl → zncl_2 + h_2s zinc sulfide react with hydrogen. Zinc Sulfide And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Sulfide And Hydrochloric Acid zinc reacts with halogens in the presence of moisture: the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide. Zn + cl₂ → zncl₂. zinc sulfide can be used to produce hydrogen sulfide in a lab by the reaction with a strong acid like. Zinc Sulfide And Hydrochloric Acid.
From hydrogenpotanezu.blogspot.com
Hydrogen Zinc Chloride Plus Hydrogen Sulfide Zinc Sulfide And Hydrochloric Acid this chemistry video tutorial explains how to predict the products of the. when zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): the reaction of zinc sulphide (z n s) with dilute hydrochloric acid produces zinc chloride (z n c l 2) and hydrogen sulphide.. Zinc Sulfide And Hydrochloric Acid.