Standard Base Excess . Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. A high base excess (> +2mmol/l). Base excess can either be expressed for whole blood— be(b), which does not consider the interaction of blood with the interstitial uid, or. For the purposes of this review, the reference values are 7.40 for. Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of.
from www.slideshare.net
Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. Base excess can either be expressed for whole blood— be(b), which does not consider the interaction of blood with the interstitial uid, or. Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). For the purposes of this review, the reference values are 7.40 for. The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. A high base excess (> +2mmol/l).
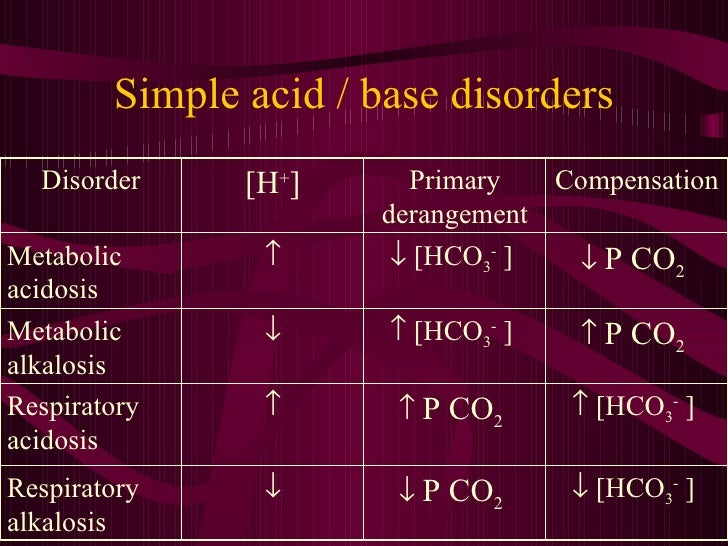
Acid / Base Balance Interpretation of Results comep oc 2010
Standard Base Excess Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Base excess can either be expressed for whole blood— be(b), which does not consider the interaction of blood with the interstitial uid, or. For the purposes of this review, the reference values are 7.40 for. Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. A high base excess (> +2mmol/l).
From www.researchgate.net
Diagrammatic representation with standard base excess in horizontal Standard Base Excess Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: For the purposes of this review, the reference values are 7.40 for. The first step is to evaluate standard base excess in relation. Standard Base Excess.
From www.researchgate.net
(PDF) Diagnostic Use of Base Excess in AcidBase Disorders Standard Base Excess For the purposes of this review, the reference values are 7.40 for. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Base excess. Standard Base Excess.
From www.semanticscholar.org
Table 1 from Evolutive standard base excess and serum lactate level in Standard Base Excess Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. A high base excess (> +2mmol/l). The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. For the. Standard Base Excess.
From www.researchgate.net
(a) Response of standard base excess to normal saline (NS) infusion in Standard Base Excess Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions. Standard Base Excess.
From www.slideserve.com
PPT Acid Base Balance and ABG Interpretation PowerPoint Presentation Standard Base Excess A high base excess (> +2mmol/l). The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. Standard base excess. Standard Base Excess.
From www.slideserve.com
PPT LABORATORIUM INTERPRETATION OF ACIDBASE & ELECTROLITES DISORDERS Standard Base Excess Base excess can either be expressed for whole blood— be(b), which does not consider the interaction of blood with the interstitial uid, or. Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. The concept of the standard base excess (sbe) was introduced to address this problem and. Standard Base Excess.
From www.researchgate.net
Annual trends in acidbase status. Standard base excess (SBE) is Standard Base Excess Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. For the purposes of this review, the reference values are 7.40 for. Standard base excess is dose of acid or alkali to return the ecf. Standard Base Excess.
From blog.agnibho.com
Acid Base Disorders Blog of Dr. Agnibho Standard Base Excess Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1. Standard Base Excess.
From www.researchgate.net
(PDF) A Graphical Tool for Arterial Blood Gas Interpretation using Standard Base Excess The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. For the purposes of this review, the reference values are 7.40 for. Standard. Standard Base Excess.
From www.slideserve.com
PPT Arterial Blood Gas Analysis PowerPoint Presentation ID670157 Standard Base Excess For the purposes of this review, the reference values are 7.40 for. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or. Standard Base Excess.
From ajemjournal-test.com.marlin-prod.literatumonline.com
The association of standard base excess upon emergency admission with Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions (. Standard Base Excess.
From www.slideserve.com
PPT Acid Base Balance and ABG Interpretation PowerPoint Presentation Standard Base Excess Base excess can either be expressed for whole blood— be(b), which does not consider the interaction of blood with the interstitial uid, or. For the purposes of this review, the reference values are 7.40 for. A high base excess (> +2mmol/l). Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a. Standard Base Excess.
From www.slideserve.com
PPT Blodgaser och syra/basstatus PowerPoint Presentation, free Standard Base Excess Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: A high base excess (> +2mmol/l). The concept of the. Standard Base Excess.
From www.youtube.com
Base Excess Explained YouTube Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). A high base excess (> +2mmol/l). Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and. Standard Base Excess.
From www.semanticscholar.org
[PDF] Diagnostic Use of Base Excess in AcidBase Disorders Semantic Standard Base Excess Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at. Standard Base Excess.
From www.nejm.org
A Critique of the Parameters Used in the Evaluation of AcidBase Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. For. Standard Base Excess.
From www.slideserve.com
PPT Lab Values and ICU Monitoring PowerPoint Presentation, free Standard Base Excess The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. For the purposes of this review, the reference. Standard Base Excess.
From www.scribd.com
Standard Base Excess and Strong Ion Theory Buffer Solution Bicarbonate Standard Base Excess Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. For the purposes of this review, the reference values are 7.40 for. A high base excess (> +2mmol/l). Base excess. Standard Base Excess.
From www.scribd.com
Base Excess Buffer Solution Major Trauma Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). A high base excess (> +2mmol/l). Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. The concept of. Standard Base Excess.
From www.researchgate.net
(PDF) Clinical utility of standard base excess in the diagnosis and Standard Base Excess A high base excess (> +2mmol/l). The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. For the purposes of this review, the reference values are 7.40 for. Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40). Standard Base Excess.
From www.mednote.dk
Acidbase balance and disorders Mednote.dk Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: A high base excess (> +2mmol/l). Standard base excess is dose of acid or alkali to return the ecf to normal. Standard Base Excess.
From www.nejm.org
Diagnostic Use of Base Excess in AcidBase Disorders NEJM Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: For the purposes of this review, the reference values are 7.40 for. A high base excess (> +2mmol/l). Base excess (be). Standard Base Excess.
From www.slideserve.com
PPT LABORATORIUM INTERPRETATION OF ACIDBASE & ELECTROLITES DISORDERS Standard Base Excess Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. A high base excess (> +2mmol/l). For the purposes of this. Standard Base Excess.
From www.researchgate.net
(PDF) Clinical utility of standard base excess in the diagnosis and Standard Base Excess Base excess (be) the base excess is another surrogate marker of metabolic acidosis or alkalosis: Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. Base excess can either be expressed for whole blood— be(b),. Standard Base Excess.
From www.slideshare.net
Acid / Base Balance Interpretation of Results comep oc 2010 Standard Base Excess The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. Base. Standard Base Excess.
From www.semanticscholar.org
Table 2 from Diagnostic Use of Base Excess in AcidBase Disorders Standard Base Excess Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at the actual. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). For. Standard Base Excess.
From www.slideserve.com
PPT ABG INTERPRETATION PowerPoint Presentation, free download ID Standard Base Excess A high base excess (> +2mmol/l). For the purposes of this review, the reference values are 7.40 for. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). Base excess can either be expressed for whole blood— be(b), which does not consider the interaction of blood. Standard Base Excess.
From www.researchgate.net
Analysis of various acidbase disturbances using standard base excess Standard Base Excess The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. A high base excess (> +2mmol/l). For the. Standard Base Excess.
From www.researchgate.net
4 QUADRANT GRAPHICAL TOOL FOR ABG USING Std Base Excess VS Modified Standard Base Excess Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Standard base excess is dose of acid or alkali to return the ecf. Standard Base Excess.
From www.youtube.com
ABG Base Excess and Deficit YouTube Standard Base Excess The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. For the purposes of this review, the reference values are 7.40 for. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table. Standard Base Excess.
From twitter.com
NEJM on Twitter "To diagnose an acidbase disorder, a threestep Standard Base Excess A high base excess (> +2mmol/l). Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c,. Standard Base Excess.
From derangedphysiology.com
Standard base excess Deranged Physiology Standard Base Excess The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Base excess can either be expressed for whole blood— be(b), which does not. Standard Base Excess.
From www.slideserve.com
PPT AcidBase Disturbance PowerPoint Presentation, free download ID Standard Base Excess Standard base excess is dose of acid or alkali to return the ecf to normal ph (7.40) under standard conditions ( at 37c at a pco2 of. Standard base excess is the concentration of titrable base when the blood is titrated back to a normal plasma ph of 7.40, at a normal pco2 ( 40 mmhg) and 37° c, at. Standard Base Excess.
From www.slideserve.com
PPT Lab Values and ICU Monitoring PowerPoint Presentation, free Standard Base Excess The concept of the standard base excess (sbe) was introduced to address this problem and is defined as the quantity of strong acid or base. For the purposes of this review, the reference values are 7.40 for. Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. Base. Standard Base Excess.
From twitter.com
Intensive Care Medicine on Twitter "Understanding base excess 🧪 Standard Base Excess A high base excess (> +2mmol/l). Base excess (be) = amount of titratable acid in mmol/l needed to titrate one litre of blood to a ph of 7.4. The first step is to evaluate standard base excess in relation to ph and p co2 (figure 1 and figure 2 and table 1). For the purposes of this review, the reference. Standard Base Excess.