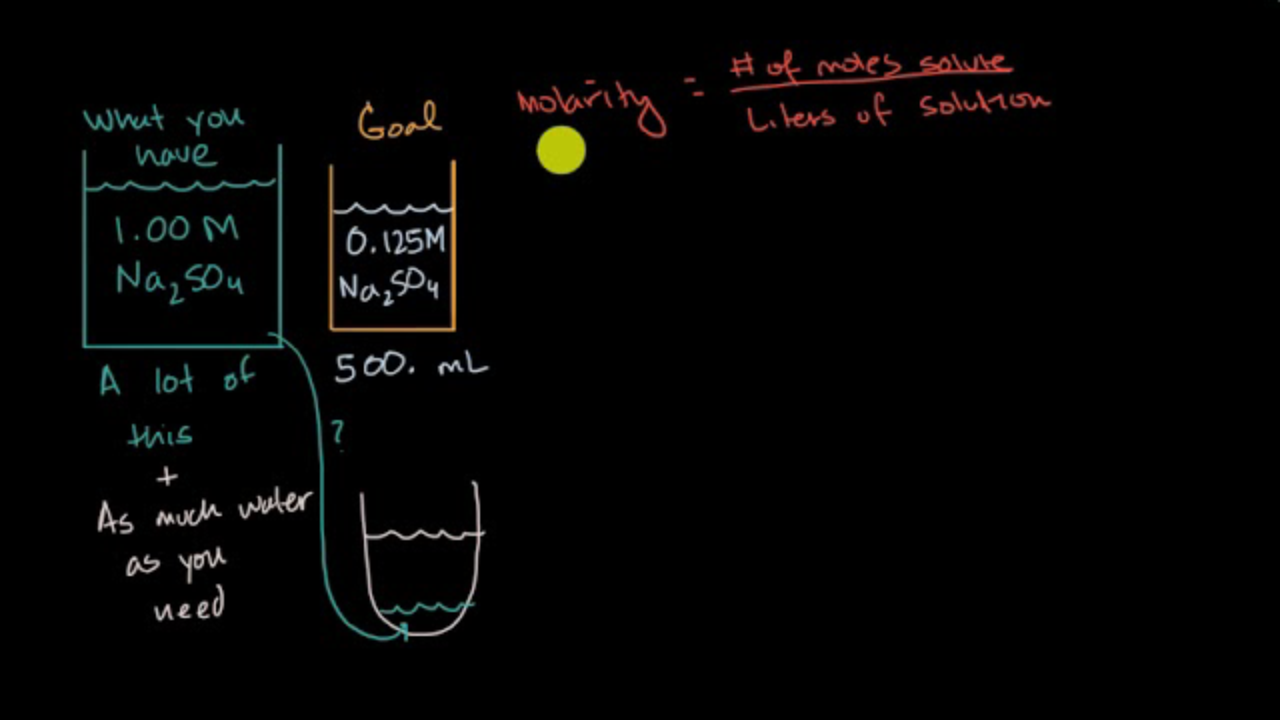
Dilution Equation Meaning . this yields the most general form of the dilution equation: to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution of solutions. Concentration is the removal of solvent, which. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more.
from fr.thptnganamst.edu.vn
Concentration is the removal of solvent, which. dilution of solutions. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Of course, the resulting solution is. this yields the most general form of the dilution equation: state whether the concentration of a solution is directly or indirectly proportional to its volume. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
Découvrir 184+ imagen dilution formule fr.thptnganamst.edu.vn
Dilution Equation Meaning Dilution is a process used to lower the concentration of the original solution by adding more. state whether the concentration of a solution is directly or indirectly proportional to its volume. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more. this yields the most general form of the dilution equation: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Of course, the resulting solution is. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. dilution of solutions.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Equation Meaning state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution of solutions. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. dilution is the process of adding. Dilution Equation Meaning.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Equation Meaning to dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Dilution is a process. Dilution Equation Meaning.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Equation Meaning dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration. Dilution Equation Meaning.
From ceonkpsw.blob.core.windows.net
Dilution Amount Meaning at Ellen Perez blog Dilution Equation Meaning state whether the concentration of a solution is directly or indirectly proportional to its volume. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Of course, the resulting solution is. dilution of. Dilution Equation Meaning.
From cejioehy.blob.core.windows.net
Dilute Meaning In Chemistry at Benjamin Hack blog Dilution Equation Meaning state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. Dilution is a process used to lower the concentration of the original solution by adding more. Of course, the resulting solution is. dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Equation Meaning.
From ar.inspiredpencil.com
Dilution Equation Dilution Equation Meaning \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. to dilute a solution means to add more solvent without the addition of more solute. state whether the concentration of a solution is directly or. Dilution Equation Meaning.
From www.slideserve.com
PPT 03 Concentration DILUTIONS PowerPoint Presentation, free Dilution Equation Meaning state whether the concentration of a solution is directly or indirectly proportional to its volume. this yields the most general form of the dilution equation: dilution of solutions. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the. Dilution Equation Meaning.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Equation Meaning to dilute a solution means to add more solvent without the addition of more solute. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Dilution is a process used to lower the concentration of the. Dilution Equation Meaning.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation Meaning dilution of solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. this yields the most general form of the dilution equation: Dilution is a process used to lower the concentration of the original solution by adding more. \[c_1v_1=c_2v_2\] to summarize, c stands for. Dilution Equation Meaning.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Equation Meaning Dilution is a process used to lower the concentration of the original solution by adding more. dilution of solutions. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. to dilute a solution means to add more solvent without the addition of more solute. \[c_1v_1=c_2v_2\] to summarize, c stands. Dilution Equation Meaning.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Equation Meaning this yields the most general form of the dilution equation: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution of solutions. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. to dilute a solution means to add more. Dilution Equation Meaning.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Equation Meaning dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Of course, the resulting solution is. dilution. Dilution Equation Meaning.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Equation Meaning to dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which. dilution of solutions. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Of course, the resulting solution is. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration. Dilution Equation Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Equation Meaning dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. dilution of solutions. Concentration is the removal of solvent, which. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2. Dilution Equation Meaning.
From www.slideshare.net
Molarity and dilution Dilution Equation Meaning this yields the most general form of the dilution equation: to dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which. state whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is. Dilution is a process. Dilution Equation Meaning.
From www.slideserve.com
PPT Part 2 Dilution Ventilation (General Ventilation) PowerPoint Dilution Equation Meaning dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more. dilution of solutions. Of course, the resulting solution. Dilution Equation Meaning.
From cepymljv.blob.core.windows.net
Dilution Lab Microbiology at Marvin Bilodeau blog Dilution Equation Meaning Dilution is a process used to lower the concentration of the original solution by adding more. dilution of solutions. Concentration is the removal of solvent, which. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of adding a solvent to a solution to reduce the concentration of. Dilution Equation Meaning.
From www.chegg.com
Use the dilution equation (MconcV conc = MdilVdil) Dilution Equation Meaning Of course, the resulting solution is. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution of solutions.. Dilution Equation Meaning.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Dilution Equation Meaning state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more. dilution of solutions. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before. Dilution Equation Meaning.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Dilution Equation Meaning Concentration is the removal of solvent, which. state whether the concentration of a solution is directly or indirectly proportional to its volume. this yields the most general form of the dilution equation: dilution of solutions. Of course, the resulting solution is. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of. Dilution Equation Meaning.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Equation Meaning state whether the concentration of a solution is directly or indirectly proportional to its volume. to dilute a solution means to add more solvent without the addition of more solute. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript. Dilution Equation Meaning.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation Meaning \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. to dilute a solution means to add more solvent without the addition of more solute. this yields the most general form of the dilution equation:. Dilution Equation Meaning.
From fr.thptnganamst.edu.vn
Découvrir 184+ imagen dilution formule fr.thptnganamst.edu.vn Dilution Equation Meaning to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution of solutions. . Dilution Equation Meaning.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation Dilution Equation Meaning this yields the most general form of the dilution equation: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. dilution of solutions. Of course, the resulting solution. Dilution Equation Meaning.
From exodwyspp.blob.core.windows.net
Dilution Water Calculator at Mary Guevara blog Dilution Equation Meaning dilution of solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. this yields the most general form of the dilution equation: Dilution is a process used to lower the concentration of the original solution by adding more. Of course, the resulting solution is. to dilute a solution means to. Dilution Equation Meaning.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Equation Meaning to dilute a solution means to add more solvent without the addition of more solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more. Concentration is the removal of solvent, which. dilution of solutions.. Dilution Equation Meaning.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Equation Meaning dilution of solutions. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of. Dilution Equation Meaning.
From exovmtirc.blob.core.windows.net
Dilution Vs. Absorption at Charlie Goings blog Dilution Equation Meaning this yields the most general form of the dilution equation: dilution of solutions. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. state whether the concentration of a solution is directly or indirectly. Dilution Equation Meaning.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Equation Meaning to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. this yields the most general form of the dilution equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a. Dilution Equation Meaning.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Equation Meaning this yields the most general form of the dilution equation: state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution of solutions. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of. Dilution Equation Meaning.
From www.slideserve.com
PPT Molarity & Dilution PowerPoint Presentation, free download ID Dilution Equation Meaning Of course, the resulting solution is. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. Dilution Equation Meaning.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Equation Meaning dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a. Dilution Equation Meaning.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution Equation Meaning Concentration is the removal of solvent, which. dilution of solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Of course, the resulting solution is. to dilute a solution means to. Dilution Equation Meaning.
From exoxgkatn.blob.core.windows.net
Dilution Problems With Answers at Michael Keller blog Dilution Equation Meaning dilution of solutions. Of course, the resulting solution is. this yields the most general form of the dilution equation: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more. \[c_1v_1=c_2v_2\] to summarize, c stands for. Dilution Equation Meaning.
From www.scribd.com
Dilution (Equation) Wikipedia PDF Solution Chemistry Dilution Equation Meaning Of course, the resulting solution is. this yields the most general form of the dilution equation: Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution of solutions. dilution is the process of adding a solvent to a solution to reduce the. Dilution Equation Meaning.